Effects of nicotine versus placebo e-cigarette use on symptom relief during initial tobacco abstinence
- PMID: 28650184
- PMCID: PMC5546939
- DOI: 10.1037/pha0000134
Effects of nicotine versus placebo e-cigarette use on symptom relief during initial tobacco abstinence
Abstract
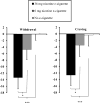
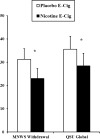
Because electronic cigarettes (e-cigs) containing nicotine may relieve smoking abstinence symptoms similar to nicotine replacement therapy medication, we used within-subjects designs to test these effects with a first-generation e-cig in nonquitting and quitting smokers. In Study 1, 28 nontreatment-seeking smokers abstained overnight prior to each of 3 sessions. Minnesota Nicotine Withdrawal Scale (MNWS) withdrawal (and craving item) relief was assessed following 4 exposures (each 10 puffs) over 2 hr to e-cigs that either did (36 mg/ml) or did not (i.e., placebo, 0 mg/ml) contain nicotine or after no e-cig. Relief was greater after nicotine versus placebo e-cig (p < .05) but not after placebo versus no e-cig, showing relief was due to nicotine per se and not simple e-cig use behavior. Using a crossover design in Study 2, smokers preparing to quit soon engaged in 2 experimental 4-day quit periods on separate weeks. In weeks 1 and 3, all received a nicotine or placebo e-cig on Monday to use ad libitum while trying to abstain from smoking on Tuesday through Friday. (Week 2 involved resumption of ad libitum smoking.) MNWS and Questionnaire of Smoking Urges (QSU) craving were assessed at daily visits following 24-hr abstinence. Of 17 enrolled, 12 quit for ≥24 hr at least once, allowing test of relief because of e-cig use on quit days. Withdrawal and craving were reduced because of nicotine versus placebo e-cig use (both p < .05). In sum, compared with placebo e-cigs, nicotine e-cigs can relieve smoking abstinence symptoms, perhaps in a manner similar to Food and Drug Administration-approved nicotine replacement therapy products, although much more research with larger samples is needed. (PsycINFO Database Record
(c) 2017 APA, all rights reserved).
Conflict of interest statement
DISCLOSURES
No author has any potential conflicts of interest to report.
Figures
Similar articles
-
Effectiveness of Non-Nicotinic E-Cigarettes to Reduce Cue- and Abstinence-Induced Cigarette Craving in Non-Treatment Seeking Daily Dependent Smokers.Psychopharmacology (Berl). 2021 Jun;238(6):1461-1472. doi: 10.1007/s00213-021-05772-4. Epub 2021 Jan 30. Psychopharmacology (Berl). 2021. PMID: 33515267
-
Effectiveness of the electronic cigarette: An eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints.Int J Environ Res Public Health. 2014 Oct 29;11(11):11220-48. doi: 10.3390/ijerph111111220. Int J Environ Res Public Health. 2014. PMID: 25358095 Free PMC article. Clinical Trial.
-
Electronic cigarette use is not associated with quitting of conventional cigarettes in youth smokers.Pediatr Res. 2017 Jul;82(1):14-18. doi: 10.1038/pr.2017.80. Epub 2017 May 24. Pediatr Res. 2017. PMID: 28355200
-
[Smoking reduction and temporary abstinence: new approaches for smoking cessation].J Mal Vasc. 2003 Dec;28(5):293-300. J Mal Vasc. 2003. PMID: 14978435 Review. French.
-
A fresh look at tobacco harm reduction: the case for the electronic cigarette.Harm Reduct J. 2013 Oct 4;10:19. doi: 10.1186/1477-7517-10-19. Harm Reduct J. 2013. PMID: 24090432 Free PMC article. Review.
Cited by
-
The Role of Nicotine and Flavor in the Abuse Potential and Appeal of Electronic Cigarettes for Adult Current and Former Cigarette and Electronic Cigarette Users: A Systematic Review.Nicotine Tob Res. 2022 Aug 6;24(9):1332-1343. doi: 10.1093/ntr/ntac073. Nicotine Tob Res. 2022. PMID: 35305014 Free PMC article.
-
Distinct influences of nicotine and sensorimotor stimuli on reducing cravings to smoke and vape among dual users.Addict Behav. 2021 Nov;122:107051. doi: 10.1016/j.addbeh.2021.107051. Epub 2021 Jul 15. Addict Behav. 2021. PMID: 34303118 Free PMC article.
-
Appeal, subjective effects, and relative reinforcing effects of JUUL that vary in flavor and nicotine content.Exp Clin Psychopharmacol. 2021 Jun;29(3):279-287. doi: 10.1037/pha0000481. Exp Clin Psychopharmacol. 2021. PMID: 34264738 Free PMC article.
-
Young adult dual combusted cigarette and e-cigarette users' anticipated responses to hypothetical e-cigarette market restrictions.Subst Use Misuse. 2019;54(12):2033-2042. doi: 10.1080/10826084.2019.1626435. Epub 2019 Jul 15. Subst Use Misuse. 2019. PMID: 31305213 Free PMC article.
-
Nicotine or expectancies? Using the balanced-placebo design to test immediate outcomes of vaping.Addict Behav. 2019 Oct;97:90-96. doi: 10.1016/j.addbeh.2019.04.026. Epub 2019 Apr 26. Addict Behav. 2019. PMID: 31174168 Free PMC article.
References
-
- Aguinis H, Gottfredson RK, Culpepper SA. Best-practice recommendations for estimating cross-level interaction effects using multilevel modeling. Journal of Management. 2013;39:1490–1528. doi: 10.1177/0149206313478188. - DOI
-
- American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. 4th. Arlington, VA: American Psychiatric Publishing; 1994.
-
- Beard E, West R, Michie S, Brown J. Association between electronic cigarette use and changes in quit attempts, success of quit attempts, use of smoking cessation pharmacotherapy, and use of stop smoking services in England: time series analysis of population trends. British Medical Journal. 2016;354:i4645. doi: 10.1136/bmj.i4645. - DOI - PubMed
-
- Cleophas TJM. Cross-over studies: a modified analysis with more power. Clinical Pharmacology & Therapeutics. 1993;53:515–520. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


