Cutaneous Adverse Events in the Randomized, Double-Blind, Active-Comparator DECIDE Study of Daclizumab High-Yield Process Versus Intramuscular Interferon Beta-1a in Relapsing-Remitting Multiple Sclerosis
- PMID: 27251051
- PMCID: PMC4939160
- DOI: 10.1007/s12325-016-0353-2
Cutaneous Adverse Events in the Randomized, Double-Blind, Active-Comparator DECIDE Study of Daclizumab High-Yield Process Versus Intramuscular Interferon Beta-1a in Relapsing-Remitting Multiple Sclerosis
Abstract
Introduction: Cutaneous adverse events (AEs) have been observed in clinical studies of daclizumab high-yield process (HYP) in relapsing-remitting multiple sclerosis (RRMS). Here, we report cutaneous AEs observed in the randomized, double-blind, active-comparator DECIDE study (ClinicalTrials.gov identifier, NCT01064401).
Methods: DECIDE was a randomized, double-blind, active-controlled phase 3 study of daclizumab HYP 150 mg subcutaneous every 4 weeks versus interferon (IFN) beta-1a 30 mcg intramuscular (IM) once weekly in RRMS. Treatment-emergent AEs were classified and recorded by investigators. Investigators also assessed the severity of each AE, and whether it met the criteria for a serious AE. Cutaneous AEs were defined as AEs coded to the Medical Dictionary for Regulatory Activities System Organ Class of skin and subcutaneous tissue disorders. The incidence, severity, onset, resolution, and management of AEs were analyzed by treatment group.
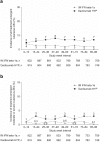
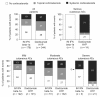
Results: Cutaneous AEs were reported in 37% of daclizumab HYP-treated patients and 19% of IFN beta-1a-treated patients. The most common investigator-reported cutaneous AEs with daclizumab HYP were rash (7%) and eczema (4%). Most patients with cutaneous AEs remained on treatment (daclizumab HYP, 81%; IM IFN beta-1a, 90%) and had events that were mild or moderate (94% and 98%) and subsequently resolved (78% and 82%). Most patients with cutaneous AEs did not require treatment with corticosteroids or were treated with topical corticosteroids (daclizumab HYP, 73%; IM IFN beta-1a, 81%). Serious cutaneous AEs were reported in 14 (2%) daclizumab HYP patients and one (<1%) IM IFN beta-1a patient.
Conclusion: There was an increased risk of cutaneous AEs with daclizumab HYP. While physicians should be aware of the potential for serious cutaneous AEs, the typical cutaneous AEs were mild-to-moderate in severity, manageable, and resolved over time.
Funding: Biogen and AbbVie Biotherapeutics Inc.
Trial registration: ClinicalTrials.gov identifier, NCT01064401.
Keywords: Cutaneous events; Daclizumab high-yield process; Dermatology; Interferon beta; Neurology; Relapsing-remitting multiple sclerosis; Safety.
Figures
Similar articles
-
Daclizumab HYP versus Interferon Beta-1a in Relapsing Multiple Sclerosis.N Engl J Med. 2015 Oct 8;373(15):1418-28. doi: 10.1056/NEJMoa1501481. N Engl J Med. 2015. PMID: 26444729 Clinical Trial.
-
Impact of daclizumab versus interferon beta-1a on patient-reported outcomes in relapsing-remitting multiple sclerosis.Mult Scler Relat Disord. 2017 Jan;11:18-24. doi: 10.1016/j.msard.2016.11.005. Epub 2016 Nov 13. Mult Scler Relat Disord. 2017. PMID: 28104250 Clinical Trial.
-
Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial.Lancet. 2013 Jun 22;381(9884):2167-75. doi: 10.1016/S0140-6736(12)62190-4. Epub 2013 Apr 4. Lancet. 2013. PMID: 23562009 Clinical Trial.
-
Daclizumab: A Review in Relapsing Multiple Sclerosis.Drugs. 2017 Mar;77(4):447-458. doi: 10.1007/s40265-017-0708-2. Drugs. 2017. PMID: 28211007 Review.
-
Daclizumab for relapsing remitting multiple sclerosis.Cochrane Database Syst Rev. 2013 Dec 23;2013(12):CD008127. doi: 10.1002/14651858.CD008127.pub4. Cochrane Database Syst Rev. 2013. PMID: 24363032 Free PMC article. Review.
Cited by
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD011381. doi: 10.1002/14651858.CD011381.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174776 Free PMC article. Review.
-
Adverse effects of immunotherapies for multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 30;11(11):CD012186. doi: 10.1002/14651858.CD012186.pub2. Cochrane Database Syst Rev. 2023. PMID: 38032059 Review.
-
Safety and efficacy of daclizumab beta in patients with relapsing multiple sclerosis in a 5-year open-label study (EXTEND): final results following early termination.Ther Adv Neurol Disord. 2021 Feb 26;14:1756286420987941. doi: 10.1177/1756286420987941. eCollection 2021. Ther Adv Neurol Disord. 2021. PMID: 33737954 Free PMC article.
-
Long-term safety and efficacy of daclizumab beta in relapsing-remitting multiple sclerosis: 6-year results from the SELECTED open-label extension study.J Neurol. 2020 Oct;267(10):2851-2864. doi: 10.1007/s00415-020-09835-y. Epub 2020 May 25. J Neurol. 2020. PMID: 32451615 Free PMC article. Clinical Trial.
-
Case Reports of DRESS Syndrome and Symptoms Consistent with DRESS Syndrome Following Treatment with Recently Marketed Monoclonal Antibodies.Autoimmune Dis. 2019 Jun 9;2019:7595706. doi: 10.1155/2019/7595706. eCollection 2019. Autoimmune Dis. 2019. PMID: 31308976 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous


