Human ex-vivo action potential model for pro-arrhythmia risk assessment
- PMID: 27235787
- PMCID: PMC5042841
- DOI: 10.1016/j.vascn.2016.05.016
Human ex-vivo action potential model for pro-arrhythmia risk assessment
Abstract
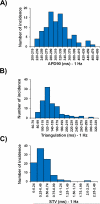
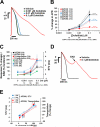
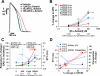
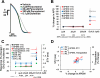
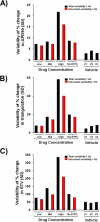
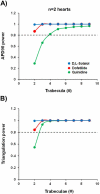
While current S7B/E14 guidelines have succeeded in protecting patients from QT-prolonging drugs, the absence of a predictive paradigm identifying pro-arrhythmic risks has limited the development of valuable drug programs. We investigated if a human ex-vivo action potential (AP)-based model could provide a more predictive approach for assessing pro-arrhythmic risk in man. Human ventricular trabeculae from ethically consented organ donors were used to evaluate the effects of dofetilide, d,l-sotalol, quinidine, paracetamol and verapamil on AP duration (APD) and recognized pro-arrhythmia predictors (short-term variability of APD at 90% repolarization (STV(APD90)), triangulation (ADP90-APD30) and incidence of early afterdepolarizations at 1 and 2Hz to quantitatively identify the pro-arrhythmic risk. Each drug was blinded and tested separately with 3 concentrations in triplicate trabeculae from 5 hearts, with one vehicle time control per heart. Electrophysiological stability of the model was not affected by sequential applications of vehicle (0.1% dimethyl sulfoxide). Paracetamol and verapamil did not significantly alter anyone of the AP parameters and were classified as devoid of pro-arrhythmic risk. Dofetilide, d,l-sotalol and quinidine exhibited an increase in the manifestation of pro-arrhythmia markers. The model provided quantitative and actionable activity flags and the relatively low total variability in tissue response allowed for the identification of pro-arrhythmic signals. Power analysis indicated that a total of 6 trabeculae derived from 2 hearts are sufficient to identify drug-induced pro-arrhythmia. Thus, the human ex-vivo AP-based model provides an integrative translational assay assisting in shaping clinical development plans that could be used in conjunction with the new CiPA-proposed approach.
Keywords: Action potential; Drug discovery and development; Human heart; Pro-arrhythmia assessment; Ventricular trabeculae.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Quantitative Comparison of Effects of Dofetilide, Sotalol, Quinidine, and Verapamil between Human Ex vivo Trabeculae and In silico Ventricular Models Incorporating Inter-Individual Action Potential Variability.Front Physiol. 2017 Aug 18;8:597. doi: 10.3389/fphys.2017.00597. eCollection 2017. Front Physiol. 2017. PMID: 28868038 Free PMC article.
-
Action Potential Recording and Pro-arrhythmia Risk Analysis in Human Ventricular Trabeculae.Front Physiol. 2018 Jan 5;8:1109. doi: 10.3389/fphys.2017.01109. eCollection 2017. Front Physiol. 2018. PMID: 29354071 Free PMC article.
-
Review of the predictive value of the Langendorff heart model (Screenit system) in assessing the proarrhythmic potential of drugs.J Pharmacol Toxicol Methods. 2004 May-Jun;49(3):171-81. doi: 10.1016/j.vascn.2004.03.008. J Pharmacol Toxicol Methods. 2004. PMID: 15172013 Review.
-
Selective block of IKs plays a significant role in MAP triangulation induced by IKr block in isolated rabbit heart.J Pharmacol Toxicol Methods. 2008 Jul-Aug;58(1):32-40. doi: 10.1016/j.vascn.2008.05.129. Epub 2008 Jun 8. J Pharmacol Toxicol Methods. 2008. PMID: 18582585
-
Point of View: Electrophysiological Endpoints Differ When Comparing the Mode of Action of Highly Successful Anti-arrhythmic Drugs in the CAVB Dog Model With TdP.J Cardiovasc Pharmacol. 2019 Dec;74(6):499-507. doi: 10.1097/FJC.0000000000000748. J Cardiovasc Pharmacol. 2019. PMID: 31738198 Review.
Cited by
-
Oxa-Iboga alkaloids lack cardiac risk and disrupt opioid use in animal models.Nat Commun. 2024 Sep 20;15(1):8118. doi: 10.1038/s41467-024-51856-y. Nat Commun. 2024. PMID: 39304653 Free PMC article.
-
Local Control Model of a Human Ventricular Myocyte: An Exploration of Frequency-Dependent Changes and Calcium Sparks.Biomolecules. 2023 Aug 17;13(8):1259. doi: 10.3390/biom13081259. Biomolecules. 2023. PMID: 37627324 Free PMC article.
-
The importance of mechanical conditions in the testing of excitation abnormalities in a population of electro-mechanical models of human ventricular cardiomyocytes.Front Physiol. 2023 Jun 8;14:1187956. doi: 10.3389/fphys.2023.1187956. eCollection 2023. Front Physiol. 2023. PMID: 37362439 Free PMC article.
-
Generation of left ventricle-like cardiomyocytes with improved structural, functional, and metabolic maturity from human pluripotent stem cells.Cell Rep Methods. 2023 Apr 24;3(4):100456. doi: 10.1016/j.crmeth.2023.100456. eCollection 2023 Apr 24. Cell Rep Methods. 2023. PMID: 37159667 Free PMC article.
-
Functional isolation, culture and cryopreservation of adult human primary cardiomyocytes.Signal Transduct Target Ther. 2022 Jul 27;7(1):254. doi: 10.1038/s41392-022-01044-5. Signal Transduct Target Ther. 2022. PMID: 35882831 Free PMC article.
References
-
- Akar FG, Rosenbaum DS. Transmural Electrophysiological Heterogeneities Underlying Arrhythmogenesis in Heart Failure. Circulation Research. 2003;93:638–645. - PubMed
-
- Anon . ICH S7B Note for guidance on the nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals. London: May 25, 2005a. Reference CHMP/ICH/423/02. - PubMed
-
- Anon . ICH E14 Note for guidance on the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for nonantiarrhythmic drugs. London: May 25, 2005b. Reference CHMP/ICH/2/04.
-
- Brandenburger M, Wenzel J, Bogdan R, Richardt D, Nguemo F, Reppel M, et al. Organotypic slice culture from human adult ventricular myocardium. Cardiovascular Research. 2012;93:50–59. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


