Fingolimod for relapsing-remitting multiple sclerosis
- PMID: 27091121
- PMCID: PMC10401910
- DOI: 10.1002/14651858.CD009371.pub2
Fingolimod for relapsing-remitting multiple sclerosis
Abstract
Background: Fingolimod was approved in 2010 for the treatment of patients with the relapsing-remitting (RR) form of multiple sclerosis (MS). It was designed to reduce the frequency of exacerbations and to delay disability worsening. Issues on its safety and efficacy, mainly as compared to other disease modifying drugs (DMDs), have been raised.
Objectives: To assess the safety and benefit of fingolimod versus placebo, or other disease-modifying drugs (DMDs), in reducing disease activity in people with relapsing-remitting multiple sclerosis (RRMS).
Search methods: We searched the Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System (CNS) Group's Specialised Trials Register and US Food and Drug Administration reports (15 February 2016).
Selection criteria: Randomised controlled trials (RCTs) assessing the beneficial and harmful effects of fingolimod versus placebo or other approved DMDs in people with RRMS.
Data collection and analysis: We used standard methodological procedures as expected by Cochrane.
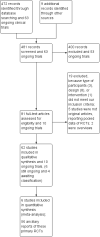
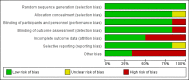
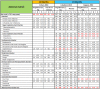
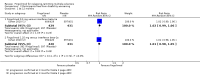
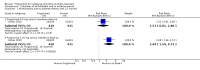
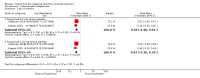
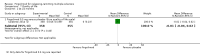
Main results: Six RCTs met our selection criteria. The overall population included 5152 participants; 1621 controls and 3531 treated with fingolimod at different doses; 2061 with 0.5 mg, 1376 with 1.25 mg, and 94 with 5.0 mg daily. Among the controls, 923 participants were treated with placebo and 698 with others DMDs. The treatment duration was six months in three, 12 months in one, and 24 months in two trials. One study was at high risk of bias for blinding, three studies were at high risk of bias for incomplete outcome reporting, and four studies were at high risk of bias for other reasons (co-authors were affiliated with the pharmaceutical company). We retrieved 10 ongoing trials; four of them have been completed.Comparing fingolimod administered at the approved dose of 0.5 mg to placebo, we found that the drug at 24 months increased the probability of being relapse-free (risk ratio (RR) 1.44, 95% confidence interval (CI) (1.28 to 1.63); moderate quality of evidence), but it might lead to little or no difference in preventing disability progression (RR 1.07, 95% CI 1.02 to 1.11; primary clinical endpoints; low quality evidence). Benefit was observed for other measures of inflammatory disease activity including clinical (annualised relapse rate): rate ratio 0.50, 95% CI 0.40 to 0.62; moderate quality evidence; and magnetic resonance imaging (MRI) activity (gadolinium-enhancing lesions): RR of being free from (MRI) gadolinium-enhancing lesions: 1.36, 95% CI 1.27 to 1.45; low quality evidence.The mean change of MRI T2-weighted lesion load favoured fingolimod at 12 and 24 months.No significant increased risk of discontinuation due to adverse events was observed for fingolimod 0.5 mg compared to placebo at six and 24 months. The risk of fingolimod discontinuation was significantly higher compared to placebo for the dose 1.25 mg at 24 months (RR 1.93, 95% CI 1.48 to 2.52).No significant increased risk of discontinuation due to serious adverse events was observed for fingolimod 0.5 mg compared to placebo at six and 24 months. A significant increased risk of discontinuation due to serious adverse events was found for fingolimod 5.0 mg (RR 2.77, 95% CI 1.04 to 7.38) compared to placebo at six months.Comparing fingolimod 0.5 mg to intramuscular interferon beta-1a, we found moderate quality evidence that the drug at one year slightly increased the number of participants free from relapse (RR 1.18, 95% CI 1.09 to 1.27) or from gadolinium-enhancing lesions (RR 1.12, 95% CI 1.05 to 1.19), and decreased the relapse rate (rate ratio 0.48, 95% CI 0.34 to 0.70). We did not detect any advantage for preventing disability progression (RR 1.02, 95% CI 0.99 to 1.06; low quality evidence). We did not detect any significant difference for MRI T2-weighted lesion load change.We found a greater likelihood of participants discontinuing fingolimod, as compared to other DMDs, due to adverse events in the short-term (six months) (RR 3.21, 95% CI 1.16 to 8.86), but there was no significant difference versus interferon beta-1a at 12 months (RR 1.51, 95% CI 0.81 to 2.80; moderate quality evidence). A higher incidence of adverse events was suggestive of the lower tolerability rate of fingolimod compared to interferon-beta 1a.Quality of life was improved in participants after switching from a different DMD to fingolimod at six months, but this effect was not found compared to placebo at 24 months.All studies were sponsored by Novartis Pharma.
Authors' conclusions: Treatment with fingolimod compared to placebo in RRMS patients is effective in reducing inflammatory disease activity, but it may lead to little or no difference in preventing disability worsening. The risk of withdrawals due to adverse events requires careful monitoring of patients over time. The evidence on the risk/benefit profile of fingolimod compared with intramuscular interferon beta-1a was uncertain, based on a low number of head-to-head RCTs with short follow-up duration. The ongoing trial results will possibly satisfy these issues.
Conflict of interest statement
Loredana La Mantia: none. Belal Firwana: none. Irene Tramacere: none. Ilaria Pacchetti: none. Roberto Palumbo: none. Graziella Filippini: none. As Co‐ordinating Editor, Dr. Filippini was excluded from the editorial process to ensure separation of the author and the editorial process. This includes all editorial decisions and related activities (e.g. Sign‐off for publication).
Figures



















Update of
- doi: 10.1002/14651858.CD009371
Similar articles
-
Teriflunomide for multiple sclerosis.Cochrane Database Syst Rev. 2016 Mar 22;3(3):CD009882. doi: 10.1002/14651858.CD009882.pub3. Cochrane Database Syst Rev. 2016. PMID: 27003123 Free PMC article. Review.
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD011381. doi: 10.1002/14651858.CD011381.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174776 Free PMC article. Review.
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2015 Sep 18;2015(9):CD011381. doi: 10.1002/14651858.CD011381.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:CD011381. doi: 10.1002/14651858.CD011381.pub3 PMID: 26384035 Free PMC article. Updated. Review.
-
Dimethyl fumarate for multiple sclerosis.Cochrane Database Syst Rev. 2015 Apr 22;2015(4):CD011076. doi: 10.1002/14651858.CD011076.pub2. Cochrane Database Syst Rev. 2015. PMID: 25900414 Free PMC article. Review.
-
Ocrelizumab for multiple sclerosis.Cochrane Database Syst Rev. 2022 May 18;5(5):CD013247. doi: 10.1002/14651858.CD013247.pub2. Cochrane Database Syst Rev. 2022. PMID: 35583174 Free PMC article. Review.
Cited by
-
The Current Landscape in the Development of Small-molecule Modulators Targeting Sphingosine-1-phosphate Receptors to Treat Neurodegenerative Diseases.Curr Top Med Chem. 2024;24(28):2431-2446. doi: 10.2174/0115680266288509240422112839. Curr Top Med Chem. 2024. PMID: 38676503 Review.
-
Alemtuzumab-induced thyroid eye disease successfully treated with a single low dose of rituximab.Eur Thyroid J. 2024 Apr 11;13(2):e230236. doi: 10.1530/ETJ-23-0236. Print 2024 Apr 1. Eur Thyroid J. 2024. PMID: 38471303 Free PMC article.
-
A comparison of clinical, utilization, and cost outcomes between oral treatments for multiple sclerosis.J Manag Care Spec Pharm. 2024 Feb 3;30(2):129-140. doi: 10.18553/jmcp.2024.30.2.129. J Manag Care Spec Pharm. 2024. PMID: 38308623 Free PMC article.
-
S1P/S1PR signaling pathway advancements in autoimmune diseases.Biomol Biomed. 2023 Nov 3;23(6):922-935. doi: 10.17305/bb.2023.9082. Biomol Biomed. 2023. PMID: 37504219 Free PMC article. Review.
-
Protein Phosphatase 2A: Role in T Cells and Diseases.J Immunol Res. 2023 May 17;2023:4522053. doi: 10.1155/2023/4522053. eCollection 2023. J Immunol Res. 2023. PMID: 37234102 Free PMC article. Review.
References
References to studies included in this review
Calabresi 2014 {published data only}
-
- Calabresi P, Radue EW, Goodin D, Jeffery D, Kottil R, Reder A, et al. Efficacy and safety of fingolimod in patients with relapsing‐remitting multiple sclerosis (RRMS): results from an additional 24‐month double‐blind, placebo‐controlled study (freedoms II study). Abstract meeting of the 64th American Academy of Neurology Annual Meeting, 2012, New Orleans, United States. Neurology. 2012.
-
- Calabresi PA, Goodin D, Jeffery D, Kappos L, Lublin FD, Rammohan K, et al. Efficacy and safety of fingolimod versus placebo: Primary outcomes from the phase 3 FREEDOMS II study in patients with relapsing‐remitting multiple sclerosis. Abstract meeting of the 28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 2012, Lyon, France. Multiple Sclerosis. 2012; Vol. 18.
-
- Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing‐remitting multiple sclerosis (FREEDOMS II): a double‐blind, randomised, placebo‐controlled, phase 3 trial. The Lancet Neurology 2014;13:545–56. - PubMed
-
- Coyle P, Cree B, Cabre P, Inglese M, Perumal J, Meng X, et al. Fingolimod efficacy and safety in an African‐American patient subgroup from freedoms II. Abstract meeting of the 66th American Academy of Neurology Annual Meeting, AAN 2014, Philadelphia, United States. Neurology. 2014; Vol. 82.
-
- Goodin D, Jeffery D, Kappos L, Lublin F, Radue EW, Rammohan K, et al. Fingolimod reduces annualized relapse rate in patients with relapsing‐remitting multiple sclerosis: Freedoms II study subgroup analysis. Abstract meeting of the 65th American Academy of Neurology Annual Meeting, 2013, San Diego, United States. Neurology. 2013; Vol. 80.
Cohen 2010 {published data only}
-
- Barkhof F, Cohen J, Montalban X, Comi G, Auberson L, Holdbrook F, et al. Fingolimod (FTY720) reduces brain volume loss over 12 months compared with intramuscular interferon beta‐1a: subgroup analyses of TRANSFORMS data based on inflammatory disease activity. Abstract meeting of the 5th Joint Triennal Congress of the European and Americas Committees for the treatment and research in Multiple Sclerosis; 2011 Oct 19–22, Amsterdam, The Netherlands. Multiple Sclerosis. 2011; Vol. 17.
-
- Barkhof F, Jong R, Sfikas N, Vera A, Francis G, Cohen J, TRANSFORMS study group. The influence of patient demographics, disease characteristics and treatment on brain volume loss in Trial Assessing Injectable Interferon vs FTY720 Oral in Relapsing‐Remitting Multiple Sclerosis (TRANSFORMS), a phase 3 study of fingolimod in multiple sclerosis. Multiple Sclerosis 2014;20(13):1704‐13. - PubMed
-
- Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral Fingolimod or intramuscular interferon for relapsing multiple sclerosis. New England Journal of Medicine 2010;362(5):402‐15. - PubMed
-
- Hartung H, Barkhof F, Comi G, Kappos L, Khatri B, Montalban X, et al. Relationship between early disease activity and long‐term clinical outcome: results from the phase 3 TRANSFORMS study extension at 4.5 years in relapsing‐remitting multiple sclerosis. Abstract meeting of the twenty‐third meeting of the ENS; 2013 June 8‐11; Barcelona, Spain. Journal of Neurology. 2013; Vol. 260.
Fox 2014 {published data only}
-
- Barbato L, Schofield L, McCague K, Pestreich L, Tobias K, Malhotra M. Randomized, open‐label study to evaluate patient‐reported outcomes (PRO) with fingolimod after changing from prior disease‐modifying therapy (DMT) for relapsing multiple sclerosis (MS): EPOC study rationale and design. Abstract meeting of the 136th Annual Meeting of the American Neurological Association; 2011 Sept 25‐27; San Diego, United States. Annals of Neurology. 2011.
-
- Calkwood J, Cree B, Crayton H, Kantor D, Brian Steingo B, Barbato L, et al. Impact of a switch to fingolimod versus staying on glatiramer acetate or beta interferons on patient‐ and physician‐reported outcomes in relapsing multiple sclerosis: post hoc analyses of the EPOC trial. BMC Neurology 2014;14(220):1‐11. - PMC - PubMed
-
- Cascione M, Wynn D, Agashivala N, McCague K, Pestreich L, Schofield L, et al. Summary scores for patient‐reported outcome measures in multiple sclerosis. Baseline data from the trial to Evaluate Patient OutComes, safety and tolerability of fingolimod (EPOC). Multiple Sclerosis 2012;18:488‐9.
-
- Cascione M, Wynn D, Barbato LM, Pestreich L, Schofield L, McCague K. Randomized, open‐label study to evaluate patient‐reported outcomes with fingolimod after changing from prior disease‐modifying therapy for relapsing multiple sclerosis: EPOC study rationale and design. Journal of Medical Economics 2013;16(7):859–65. - PubMed
-
- Crayton H, Hunter S, Huffman C, Agashivala N, Schofield L, McCague K, et al. Improved quality of life after therapy change to fingolimod. Journal of Neurology 2013;260:S127.
Kappos 2006 {published data only}
-
- Antel J, Montalban X, O'Connor P, Vera A, Cremer M, Sfikas N, et al. Long‐term (7‐year) data from a phase 2 extension study of fingolimod in relapsing multiple sclerosis. Abstract meeting of The American Academy of Neurology, 64th AAN Annual Meeting; April 21 ‐ 28, 2012; New Orleans, United States. Neurology. 2012; Vol. 78.
-
- Cohen JA, Khatri B, Barkhof F, Comi G, Hartung HP, Montalban X, et al. Long‐term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. Journal of Neurology, Neurosurgery, and Psychiatry 2015 June 25 [Epub ahead of print]. - PMC - PubMed
-
- Comi G, O'Connor P, Montalban X, Antel J, Radue EW, Karlsson G, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3‐year results. Multiple Sclerosis 2009;16:197‐207. - PubMed
-
- Izquierdo G, O'Connor P, Montalban X, Rosenstiel P, Cremer M, Vera A, et al. Five‐year results from a phase 2 study of oral fingolimod in relapsing multiple sclerosis. Multiple Sclerosis 2014;20(7):877‐81. - PubMed
-
- Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. New England Journal of Medicine 2006;355(11):1124‐40. - PubMed
Kappos 2010 {published data only}
-
- Bergvall N, Sfikas N, Alsop J, Chin P, Rosensteil P, Kappos L. Consequences of different definitions of confirmed disability progression across randomised trials of MS therapies. Multiple Sclerosis 2012;18(4):473‐4.
-
- Camu W, Thouvenot E, Meinel M, Sfikas N, Chin P, Piani‐Meier D, et al. Influence of baseline clinical and demographic characteristics on disease evolution in the phase 3 FREEDOMS study in patients with relapsing‐remitting multiple sclerosis. Abtract meetings of the ECTRIMS 18th Annual Conference on Rehabilitation, 2013, Copenhagen, Denmark. Multiple Sclerosis. 2013; Vol. 19.
-
- Chin P, Rosenstiel P, Haering D, Francis G, Kappos L. Fingolimod leads to early clinical and MRI benefits in relapsing‐remitting multiple sclerosis. Abstract meeting of the twenty‐third ENS, 2013, Spain. Journal of Neurology. 2013.
-
- Cutter G, Chin P, Francis G, Meng X, Hashmonay R, Lublin F. Relapse is associated with residual deficits in relapsing‐remitting multiple sclerosis: Analysis of freedoms data. Abstract meeting, The American Academy of Neurology's 65th AAN Annual Meeting, 2013, San Diego, United States. Neurology. 2013; Vol. 80.
-
- Devonshire V, Havrdova E, Radue EW, O'Connor P, Zhang‐Auberson L, Agoropoulou C, et al. Relapse and disability outcomes in patients with multiple sclerosis treated with Fingolimod: subgroup analyses of the double‐blind, randomised, placebo‐controlled FREEDOMS study. The Lancet Neurology 2012;11:420–8. - PubMed
Saida 2012 {published data only}
-
- Kira J, Itoyama Y, Kikuchi S, Hao Q, Kurosawa T, Nagato K, et al. Oral fingolimod (FTY720) in Japanese patients with relapsing multiple sclerosis: Results of a 12‐month, phase 2 extension study. Multiple Sclerosis 2011;17(10):S193.
-
- Saida T, Kikuchi S, Itoyama Y, Hao Q, Kurosawa T, Nagato K, et al. A randomized, controlled trial of fingolimod (FTY720) in Japanese patients with multiple sclerosis. Multiple Sclerosis 2012;18:1269–77. [PUBMED: 22354739] - PubMed
-
- Saida T, Kikuchi S, Itoyama Y, Hao Q, Kurosawa T, Nagato K, et al. Oral fingolimod (FTY720) in Japanese patients with relapsing multiple sclerosis: Results of a 6‐month, randomised, double‐blind, placebo‐controlled, phase 2 study. Multiple Sclerosis 2011;17:S418‐9.
References to studies excluded from this review
Boulton 2013 {published data only}
-
- Boulton C, David OJ, Meiser K, Schmouder R. Tolerability and pulmonary pharmacodynamic effects during treatment initiation of once‐daily oral fingolimod in subjects with moderate asthma. Clinical Pharmacology in Drug Development 2013;2(1):2‐10. - PubMed
Chinea 2014 {published data only}
-
- Chinea A, Alvarenga R, Tomic D, DiBernardo A, Meng X, Hawker K. Efficacy and safety of fingolimod in hispanic patients: Pooled data from three phase 3 clinical trials. Neurology 2014;82:10.
Comi 2013 {published data only}
-
- Comi G, Gold R, Kappos L, Rosenstiel P, Sinha A, Tomic D. Relapse and safety outcomes in patients who transitioned from glatiramer acetate or interferon (beta) to fingolimod in the open‐label FIRST study. Multiple Sclerosis 2013;19:205.
Francis 2014 {published data only}
-
- Francis G, Kappos L, O’Connor P, Collins W, Tang D, Mercier F, et al. Temporal profile of lymphocyte counts and relationship with infections with fingolimod therapy. Multiple Sclerosis 2014;20(4):471–80. - PubMed
Gold 2014 {published data only}
Green 2013 {published data only}
-
- Green A, Sergott R, Bennett L, Hamilton S, Costello F, Dahlke F, et al. Fingolimod for the treatment of acute optic neuritis: design of a phase 2 study. Multiple Sclerosis 2013;19:238‐9.
Havla 2013 {published data only}
-
- Havla J, Tackenberg B, Hellwig K, Meinl I, Krumbholz M, Seitz F, et al. Fingolimod reduces recurrence of disease activity after natalizumab withdrawal in multiple sclerosis. Journal of Neurology 2013;260(5):1382‐7. - PubMed
Kappos 2014 {published data only}
-
- Kappos L, Zhang L, Francis AG, Cohen J. Fingolimod in relapsing multiple sclerosis: An integrated analysis of safety findings. Multiple Sclerosis and Related Disorders 2014;3:494–504. - PubMed
Kappos 2014a {published data only}
-
- Kappos L, Radue E, Karlsson G, Zheng H, Rosenstiel P, Jeffery D. Efficacy benefits of fingolimod 0.5 mg once daily in patients previously treated with glatiramer acetate: Pooled analysis of phase 3 FREEDOMS and FREEDOMS II studies. Neurology 2014;82(10 Suppl):193.
Kappos 2015 {published data only}
-
- Kappos L, Mehling M, Arroyo R, Izquierdo G, Selmaj K, Curovic‐Perisic V, et al. Randomized trial of vaccination in fingolimod‐treated patients with multiple sclerosis. Neurology 2015;84(9):872‐9. - PubMed
Kappos 2015a {published data only}
Karlsson 2014 {published data only}
laroni 2013 {published data only}
Limmorth 2013 {published data only}
-
- Limmroth V, Hoyer S, Schuh K, Lang M, Hoffmann O, Ziemssen T. Good cardiac safety profile after fingolimod (Gilenya registered trademark) treatment initiation in patients with relapsing remitting multiple sclerosis: First interim analysis of the START study. Multiple Sclerosis. 2013; Vol. 19.
Lublin 2016 {published data only}
-
- Lublin F, Miller DH, Freedman MS, Cree BA, Wolinsky JS, Weiner H, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double‐blind, placebo‐controlled trial. Lancet 2016 Jan 27 [Epub ahead of print]. - PubMed
Nolan 2013 {published data only}
Van Lokven 2013 {published data only}
-
- Lokven T, Ortler S, Moser S, Vollmar P, Ziemssen T. Comparison of therapy efficacy and satisfaction of German relapsing remitting multiple sclerosis (RRMS) patients on baseline therapy with fingolimod‐treated patients; results of an interim analysis of two non‐interventional studies (PANGAEA and PEARL). Multiple Sclerosis. 2013; Vol. 19:252.
Vollmer 2013 a {published data only}
-
- Vollmer T, Radue E, Vermersch P, Rosenstiel P, Putzki N, Meinel M, et al. Clinical and magnetic resonance imaging (MRI) disease activity after fingolimod discontinuation. Multiple Sclerosis 2013;19:227‐8.
Zarbin 2013 {published data only}
-
- Zarbin M, Jampol L, Jager R, Reder A, Francis G, Collins W, et al. Ophthalmic evaluations in clinical studies of fingolimod (FTY720) in multiple sclerosis. Ophthalmology 2013;120(7):1432‐9. - PubMed
References to studies awaiting assessment
NCT01317004 {published data only}
-
- NCT01317004. A 6‐month, randomized, active comparator, open‐label, multi‐center study to evaluate patient outcomes, safety and tolerability of fingolimod (FTY720) 0.5 mg/day in patients with relapsing remitting multiple sclerosis who are candidates for ms therapy change from previous disease modifying therapy (EPOC). clinicaltrials.gov/ct2/show/NCT01317004?term=NCT01317004&rank=1 (accessed 22 February 2016).
NCT01333501 {published data only}
-
- NCT01333501. An 18‐month, open‐label, Rater‐blinded, randomized, multi‐center, active‐controlled, parallel‐group pilot study to assess efficacy and safety of fingolimod in comparison to interferon beta 1b in treating the cognitive symptoms associated with relapsing‐remitting multiple sclerosis and to assess possible relationship of these effects to regional brain atrophy. clinicaltrials.gov/ct2/show/study/NCT01333501?term=NCT01333501&rank=1 (accessed 22 February 2016).
NCT01534182 {published data only}
-
- NCT01534182. A 6‐month, randomized, active comparator, open‐label, multi‐center study to evaluate patient outcomes, safety and tolerability of (Fingolimod) 0.5 mg/day in patients with relapsing remitting multiple sclerosis who are candidates for multiple sclerosis (MS) therapy change from previous disease modifying therapy (DMT). clinicaltrials.gov/ct2/show/NCT01534182?term=NCT01534182&rank=1 (accessed 10 December 2015).
NCT01623596 {published data only}
-
- NCT01623596. A 12‐month, Prospective, Randomized, Active‐controlled, Open‐label Study to Evaluate the Patient Retention of Fingolimod vs. Approved First‐line Disease Modifying Therapies in Adults With Relapsing Remitting Multiple Sclerosis (PREFERMS). https://clinicaltrials.gov/ct2/show/study/NCT01623596?term=NCT01623596&r... 22 February 2016.
References to ongoing studies
EUCTR2013‐004622‐29‐IT {published data only}
-
- EUCTR2013‐004622‐29‐IT. A multicenter, randomized, open‐label study to assess the impact of natalizumab versus fingolimod on central nervous system tissue damage and recovery in active relapsing‐remitting multiple sclerosis. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=2013‐00462... (accessed 10 December 2015).
NCT01633112 {published data only}
-
- NCT01633112. A 12‐month, randomized, rater‐ and dose‐blinded study to compare the efficacy and safety of fingolimod 0.25 mg and 0.5 mg administered orally once daily with glatiramer acetate 20 mg administered subcutaneously once daily in patients with relapsing‐remitting multiple sclerosis. https://clinicaltrials.gov/ct2/show/study/NCT01633112?term=NCT01633112&r... (accessed 10 December 2015).
NCT01892722 {published data only}
-
- NCT01892722. Two‐year, double‐blind, randomised, multicenter, active‐controlled study to evaluate safety and efficacy of oral fingolimod versus interferon beta‐1a i.m. In pediatric patients with multiple sclerosis. https://clinicaltrials.gov/ct2/show/study/NCT01892722?term=NCT01892722&r... (accessed 10 December 2015).
NCT02141022 {published data only}
-
- NCT02141022. A pilot study of plasticity‐based and adaptive cognitive remediation in adults with multiple sclerosis treated with Gilenya. https://clinicaltrials.gov/ct2/show/study/NCT02141022?term=NCT02141022&r... (accessed 26 June 2015).
NCT02307838 {published data only}
-
- NCT02307838. Long‐term follow‐up at 10 years of patients enrolled in the fingolimod phase ii program in relapsing multiple sclerosis. https://clinicaltrials.gov/ct2/show/NCT02307838?term=NCT02307838&rank=1 (accessed 10 December 2015).
NCT02342704 {published data only}
-
- NCT02342704. A multicenter, randomized, open‐label study to assess the impact of Natalizumab versus fingolimod on central nervous system tissue damage and recovery in active relapsing‐remitting multiple sclerosis subjects. https://clinicaltrials.gov/ct2/show/study/NCT02342704?term=NCT02342704&r... (accessed 10 December 2015).
Additional references
AIFA 2015
-
- Agenzia Italiana del Farmaco (AIFA). [Nota Informativa Importante sul primo caso di Leucoencefalopatia Multifocale Progressiva (PML) in un paziente con sclerosi multipla in trattamento con fingolimod]. http://www.agenziafarmaco.gov.it/it/content/nota‐informativa‐importante‐... (accessed 10 December 2015).
Ali 2013
-
- Ali R, Nicholas RS, Muraro PA. Drugs in development for relapsing multiple sclerosis. Drugs 2013;73(7):623‐50. - PubMed
Alonso 2008
Antel 2012
-
- Antel J, Montalban X, O'Connor P, Vera A, Cremer M, Sfikas N, et al. Long‐term (7‐year) data from a phase 2 extension study of fingolimod in relapsing multiple sclerosis. Meeting abstracts of The American Academy of Neurology's 64th AAN Annual Meeting; 2012 April 23; New Orleans. Neurology. 2012.
Atkinson 2004
-
- Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health and Quality of Life Outcomes 2004;2:1‐13. - PMC - PubMed
Bao 2012
-
- Bao C, Zhou JH. Efficacy and safety of FTY720 in the treatment of relapsing‐remitting multiple sclerosis: A systematic review. Chinese Journal of Evidence‐Based Medicine 2012;12(4):445‐50.
Brinkmann 2002
-
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R. The immune modulator FTY720 targets sphingosine 1‐phosphate receptors. Journal of Biological Chemistry 2002;277:21453‐7. - PubMed
Cascione 2013
-
- Cascione M, Wynn D, Barbato LM, Pestreich L, Schofield L, McCague K. Randomized, open‐label study to evaluate patient‐reported outcomes with fingolimod after changing from prior disease‐modifying therapy for relapsing multiple sclerosis: EPOC study rationale and design. Journal of Medical Economics 2013;16(7):859–65. - PubMed
Chun 2010
Cohen 2012
-
- Cohen JA, Reingold SC, Polman CH, Wolinsky JW, International Advisory Committee on Clinical Trials in Multiple Sclerosis. Disability outcome measures in multiple sclerosis clinicaltrials: current status and future prospects. Lancet Neurology 2012;11(5):467–76. - PubMed
Cohen 2013
Comi 2010
-
- Comi G, O'Connor P, Montalban X, Antel J, Radue EW, Karlsson G, et al. Phase II study of oral fingolimod (FTY720) in multiple sclerosis: 3‐year results. Multiple Sclerosis 2010;16(2):197‐207. - PubMed
Compston 2002
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2002;359:1221‐31. - PubMed
Compston 2008
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502‐17. - PubMed
David 2012
-
- David OJ, Kovaric GM, Schmouder RL. Clinical pharmacokinetics of fingolimod. Clinical Pharmacokinetics 2012;51(1):15‐28. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Verion 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Del Santo 2011
-
- Santo F, Maratea D, Fadda V, Trippoli S, Messori A. Treatments for relapsing‐remitting multiple sclerosis: summarising current information by network meta‐analysis. European Journal of Clinical Pharmacology 2011;68:441‐8. - PubMed
Devonshire 2012
-
- Devonshire V, Havrdova E, Radue EW, O'Connor P, Zhang‐Auberson L, Agoropoulou C, et al. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double‐blind, randomised, placebo‐controlled FREEDOMS study. The Lancet Neurology 2012;11:420‐8. - PubMed
Doggrell 2010
-
- Doggrell SA. Oral fingolimod for relapsing‐remitting multiple sclerosis. Expert Opinion on Pharmacotherapy 2010;11(10):1777‐81. - PubMed
Egger 1997
Elhami 2011
-
- Elhami SR, Mohammad K, Sahraian MA, Eftekhar H. A 20‐year incidence trend (1989‐2008) and point prevalence (March 20, 2009) of multiple sclerosis in Tehran, Iran: a population‐based study. Neuroepidemiology 2011;36(3):141‐7. - PubMed
EMA 2015
-
- European Medicines Agency. Gilenya : EPAR ‐ Summary for the public. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2... (accessed Decembre 2015). [ema.europa.eu/Find medicine/Humanmedicines/European Public Assessment Reports]
EMA 2011
-
- European Medicines Agency. Committee for medicinal products for human use (CHMP). Gylenia summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_asses... (accessed December 2015).
FDA 2013
-
- US Food, Drug Administration. Safety. http://www.fda.gov/safety/medwatch/howtoreport/ucm053087.htm (accessed December 2015).
FDA 2010
-
- US Food and Drug Administration. Gilenya (Fingolimod) Product Approval Information 2010. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... 2010; Vol. (accessed December 2015).
FDA 2015
-
- Drug Safety Communication. Gilenya (Fingolimod): Drug Safety Communication ‐ FDA Warns About Cases of Rare Brain Infection. http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhuma... (accessed December 2015).
Fox 2012
-
- Fox RJ, Rudick RA. Risk stratification and patient counselling for natalizumab in multiple sclerosis. Neurology 2012;78:436‐7. - PubMed
Freedman 2013
-
- Freedman MS. Treatment options for patients with multiple sclerosis who have a suboptimal response to Interferon‐ß therapy. European Journal of Neurology 2014;21:377‐87. - PubMed
Gajofatto 2015
Giovannoni 2015
-
- Giovannoni G, Turnera B, Gnanapavana S, Offiahc C, Schmierera K, Marta M. Is it time to target no evident disease activity(NEDA) in multiple sclerosis?. Multiple Sclerosis and Related Disorders 215;4:329–33. - PubMed
Gold 2011
-
- Gold R. Oral therapies for multiple sclerosis: A review of agents in phase III development or recently approved. CNS Drugs 2011;25(1):37‐52. - PubMed
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hillert 2012
-
- Hillert J. In the coming year we should abandon Interferons and glatiramer acetate as first line therapy for MS: no. Multiple Sclerosis 2013;19(1):26‐8. - PubMed
Hutchinson 2014
-
- Hutchinson M, Fox R, Havrdova E, Kurukulasuriya N, Sarda S, Agarwal S, et al. Efficacy and safety of BG‐12 (dimethyl fumarate) and other disease‐modifying therapies for the treatment of relapsing‐remitting multiple sclerosis: a systematic review and mixed treatment comparison. Current Medical Research and Opinion 2014;30(4):613‐27. - PubMed
Izquierdo 2013
-
- Izquierdo G, O'Connor P, Montalban X, Rosenstiel P, Cremer M, Vera A, et al. Five‐year results from a phase 2 study or oral fingolimod in relapsing multiple sclerosis. Multiple Sclerosis 2014;20:877‐81. - PubMed
Jenkinson 1999
Kappos 2015b
Koch‐Henriksen 2010
-
- Koch‐Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurology 2010;9:520–32. - PubMed
Koutsouraki 2010
-
- Koutsouraki E, Costa V, Baloyannis S. Epidemiology of multiple sclerosis in Europe: a review. International Review of Psychiatry 2010;22(1):2‐13. - PubMed
Kremenchutzky 2014
-
- Kremenchutzky M, O'Connor P, Hohlfeld R, Zhang‐Auberson L, Rosenstiel P, Meng X, et al. Impact of prior treatment status and reasons for discontinuation on the efficacy and safety of fingolimod: Subgroup analyses of the Fingolimod Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis (FREEDOMS) study. Multiple Sclerosis and Related Disorders. 2014; Vol. 3:341–9. - PubMed
Kurtzke 1983
-
- Kurtzke J. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444‐52. - PubMed
Lavery 2014
Liu 2013
-
- Liu J, Zhang C, Tao W, Liu M. Systematic review and meta‐analysis of the efficacy of sphingosine‐1‐phosphate (S1P) receptor agonist FTY720 (fingolimod) in animal models of stroke. The International Journal of Neuroscience 2013;123(3):163‐9. - PubMed
Lu 2013
-
- Lu E, Wang BW, Alwan S, Synnes A, Dahlgreen L, Sadovnick A, et al. A review of safety‐related pregnancy data surrounding the oral disease‐modifying drugs for multiple sclerosis. CNS Drugs 2014;28(2):89‐94. - PubMed
Lublin 2014
Matloubian 2004
-
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004;427:355‐60. - PubMed
Mc Donald 2001
-
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnosis criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121‐7. - PubMed
Miron 2008
-
- Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel GP. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Annals of Neurology 2008;63(1):61‐71. - PubMed
Moher 2009
Montalban 2011a
-
- Montalban X, Comi G, O'Connor P, Gold S, Vera A, Eckert B, et al. Oral fingolimod (FTY720) in relapsing multiple sclerosis: impact on health‐related quality of life in a phase II study. Multiple Sclerosis 2011;17(11):1341‐50. - PubMed
Montalban 2011b
-
- Montalban X, O'Connor P, Izquierdo G, Rosenstiel P, Cremer M, Prut L, et al. Long‐term Fingolimod (FTY720) in relapsing MS: 5‐year results from an extension of a phase II, multicentre study show a sustained low level of disease activity. Abstract meeting of the 5th Joint Triennal Congress of The European and Americas Committees for Treatment and Research in Multiple Sclerosis, 2011, Amsterdam, The Netherlands. Multiple Sclerosis. 2011; Vol. 17.
Montalban 2012
-
- Montalban X, Comi G, Antel J, O'Connor P, Vera A, Cremer M, et al. Long‐term (>7‐year) efficacy and safety data from a phase II extension study of fingolimod in relapsing multiple sclerosis. Journal of Neurology 2012;259(1):S69‐70.
Nixon 2014
Noseworthy 2000
-
- Noseworthy JH, Lucchinetti CF, Rodriguez M, Weinshenker BG. Multiple Sclerosis. New England Journal of Medicine 2000;343:938‐52. - PubMed
Novartis 2016
-
- Novartis. Novartis provides on fingolimod phase III trial in primary progressive MS (PPMS). https://www.novartis.com/news/media‐releases/novartis‐provides‐update‐fi... (accessed 18 January 2016).
O'Connor 2009
-
- O'Connor P, Comi G, Montalban X, Antel J, Radue EW, Vera A, et al. FTY720 D2201 Study Group. Oral fingolimod (FTY720) in multiple sclerosis: two‐year results of a phase II extension study. Neurology 2009;72(1):73‐9. - PubMed
Oh J 2013
-
- Oh J, O'Connor PW. Safety, tolerability and efficacy of oral therapies for relapsing‐remitting multiple sclerosis. CNS Drugs 2013;27:591‐609. - PubMed
Parfenov 2013
-
- Parfenov V, Schluep M, Du Pasquier R. Assessing risk of multiple sclerosis therapies. Journal of the Neurological Sciences 2013;15:59‐65. - PubMed
Paugh 2006
-
- Paugh S, Cassidy M, He H, Milstien S, Sim‐Selley L, Spiegel S, et al. Sphingosine and its analog, the immunosuppressant 2‐Amino‐2‐(2‐[4‐octylphenyl]ethyl)‐1,3‐propanediol, interact with the CB1 cannabinoid receptor. Molecular Pharmacology 2006;70:41–50. - PubMed
Pinschewer 2000
-
- Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, Zinkernagel RM. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. Journal of Immunology 2000;164:5761‐70. - PubMed
Pittock 2004
-
- Pittock SJ, McClelland RL, Mayr WT, Jorgensen NW, Weinshenker BG, Noseworthy J, et al. Clinical implications of benign multiple sclerosis: a 20‐year population‐based follow‐up study. Annals of Neurology 2004;56:303‐6. - PubMed
Polman 2005
-
- Polman C, Reingold S, Edan G, Filippi M, Hartung H, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Annals of Neurology 2005;58:840–6. - PubMed
Polman 2011
RevMan 2015 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2015.
Richards 2002
-
- Richards RG, Sampson FC, Beard SM, Tappenden P. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technology Assessment 2002;6(10):1‐73. [PUBMED: 12022938] - PubMed
Rotstein 2015
-
- Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL. Evaluation of no evidence of disease activity in a 7‐year longitudinal multiple sclerosis cohort. JAMA 2015;72(2):152‐8. - PubMed
Scalfari 2010
Scalfari 2013
-
- Scalfari A, Neuhaus A, Daumer M, Deluca GC, Muraro PA, Ebers GC. Early relapses, onset of progression, and late outcome in multiple sclerosis. JAMA 2013;70(2):214‐22. - PubMed
Tramacere 2015
Vickrey 1995
-
- Vickrey BG, Hays RD, Harooni R, Harooni R, Ellison GW. A health‐related quality of life measure for multiple sclerosis. Quality of Life Research 1995;4(3):187‐206. - PubMed
Warrender‐Sparkes 2015
-
- Warrender‐Sparkes M, Spelman T, Izquierdo G, Trojano M, Lugaresi A, Grand’Maison F, et al. The effect of oral immunomodulatory therapy on treatment uptake and persistence in multiple sclerosis. Multiple Sclerosis 2015 Jul 21 [Epub ahead of print]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


