Biosynthesis of Thiamin Pyrophosphate
- PMID: 26443755
- PMCID: PMC6039189
- DOI: 10.1128/ecosalplus.3.6.3.7
Biosynthesis of Thiamin Pyrophosphate
Abstract
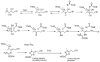
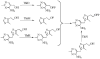
The biosynthesis of thiamin pyrophosphate (TPP) in prokaryotes, as represented by the Escherichia coli and the Bacillus subtilis pathways, is summarized in this review. The thiazole heterocycle is formed by the convergence of three separate pathways. First, the condensation of glyceraldehyde 3-phosphate and pyruvate, catalyzed by 1-deoxy-D-xylulose 5-phosphate synthase (Dxs), gives 1-deoxy-D-xylulose 5-phosphate (DXP). Next, the sulfur carrier protein ThiS-COO- is converted to its carboxyterminal thiocarboxylate in reactions catalyzed by ThiF, ThiI, and NifS (ThiF and IscS in B. subtilis). Finally, tyrosine (glycine in B. subtilis) is converted to dehydroglycine by ThiH (ThiO in B. subtilis). Thiazole synthase (ThiG) catalyzes the complex condensation of ThiS-COSH, dehydroglycine, and DXP to give a thiazole tautomer, which is then aromatized to carboxythiazole phosphate by TenI (B. subtilis). Hydroxymethyl pyrimidine phosphate (HMP-P) is formed by a complicated rearrangement reaction of 5-aminoimidazole ribotide (AIR) catalyzed by ThiC. ThiD then generates hydroxymethyl pyrimidine pyrophosphate. The coupling of the two heterocycles and decarboxylation, catalyzed by thiamin phosphate synthase (ThiE), gives thiamin phosphate. A final phosphorylation, catalyzed by ThiL, completes the biosynthesis of TPP, the biologically active form of the cofactor. This review reviews the current status of mechanistic and structural studies on the enzymes involved in this pathway. The availability of multiple orthologs of the thiamin biosynthetic enzymes has also greatly facilitated structural studies, and most of the thiamin biosynthetic and salvage enzymes have now been structurally characterized.
Figures


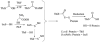
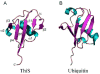
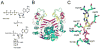
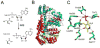


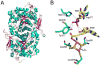
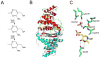

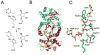
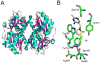
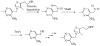

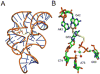
Similar articles
-
Biosynthesis of the thiazole moiety of thiamin pyrophosphate (vitamin B1).Biochemistry. 2003 Oct 28;42(42):12430-8. doi: 10.1021/bi034902z. Biochemistry. 2003. PMID: 14567704
-
Thiamin biosynthesis in prokaryotes.Arch Microbiol. 1999 Apr;171(5):293-300. doi: 10.1007/s002030050713. Arch Microbiol. 1999. PMID: 10382260 Review.
-
The biosynthesis of the thiazole phosphate moiety of thiamin: the sulfur transfer mediated by the sulfur carrier protein ThiS.Chem Biol. 2004 Oct;11(10):1373-81. doi: 10.1016/j.chembiol.2004.08.009. Chem Biol. 2004. PMID: 15489164
-
The biosynthesis of the thiazole phosphate moiety of thiamin (vitamin B1): the early steps catalyzed by thiazole synthase.J Am Chem Soc. 2004 Mar 17;126(10):3091-6. doi: 10.1021/ja039616p. J Am Chem Soc. 2004. PMID: 15012138
-
Thiamin biosynthesis: still yielding fascinating biological chemistry.Biochem Soc Trans. 2012 Jun 1;40(3):555-60. doi: 10.1042/BST20120084. Biochem Soc Trans. 2012. PMID: 22616866 Free PMC article. Review.
Cited by
-
Characterization of an Acinetobacter baumannii Monofunctional Phosphomethylpyrimidine Kinase That Is Inhibited by Pyridoxal Phosphate.Biochemistry. 2024 Feb 2:10.1021/acs.biochem.3c00640. doi: 10.1021/acs.biochem.3c00640. Online ahead of print. Biochemistry. 2024. PMID: 38306231
-
Mapping competitive pathways to terpenoid biosynthesis in Synechocystis sp. PCC 6803 using an antisense RNA synthetic tool.Microb Cell Fact. 2023 Feb 23;22(1):35. doi: 10.1186/s12934-023-02040-2. Microb Cell Fact. 2023. PMID: 36823631 Free PMC article.
-
L-tyrosine-bound ThiH structure reveals C-C bond break differences within radical SAM aromatic amino acid lyases.Nat Commun. 2022 Apr 27;13(1):2284. doi: 10.1038/s41467-022-29980-4. Nat Commun. 2022. PMID: 35477710 Free PMC article.
-
The Multifaceted Bacterial Cysteine Desulfurases: From Metabolism to Pathogenesis.Antioxidants (Basel). 2021 Jun 23;10(7):997. doi: 10.3390/antiox10070997. Antioxidants (Basel). 2021. PMID: 34201508 Free PMC article. Review.
-
Bacillus subtilis, the model Gram-positive bacterium: 20 years of annotation refinement.Microb Biotechnol. 2018 Jan;11(1):3-17. doi: 10.1111/1751-7915.13043. Microb Biotechnol. 2018. PMID: 29280348 Free PMC article.
References
-
- Wrenger C, Eschbach ML, Muller IB, Laun NP, Begley TP, Walter RD. Vitamin B1 de novo synthesis in the human malaria parasite Plasmodium falciparum depends on external provision of 4-amino-5-hydroxymethyl-2-methylpyrimidine. Biol Chem. 2006;387:41–51. - PubMed
-
- Lawhorn BG, Mehl RA, Begley TP. Biosynthesis of the thiamin pyrimidine: the reconstitution of a remarkable rearrangement reaction. Org Biomol Chem. 2004;2:2538–2546. - PubMed
-
- Settembre EC, Dorrestein PC, Park JH, Augustine AM, Begley TP, Ealick SE. Structural and mechanistic studies on ThiO, a glycine oxidase essential for thiamin biosynthesis in Bacillus subtilis. Biochemistry. 2003;42:2971–2981. - PubMed
-
- Toms AV, Haas AL, Park JH, Begley TP, Ealick SE. Structural characterization of the regulatory proteins TenA and TenI from Bacillus subtilis and identification of TenA as a thiaminase II. Biochemistry. 2005;44:2319–2329. - PubMed
-
- Himmeldirk K, Sayer BG, Spenser ID. Comparative biogenetic anatomy of vitamin B1: a 13C NMR investigation of the biosynthesis of thiamin in Escherichia coli and in Saccharomyces cerevisiae. J Am Chem Soc. 1998;120:3581–3589.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases


