Ion-pumping microbial rhodopsins
- PMID: 26442282
- PMCID: PMC4585134
- DOI: 10.3389/fmolb.2015.00052
Ion-pumping microbial rhodopsins
Abstract
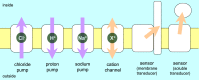
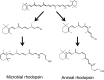
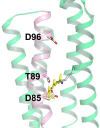
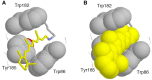
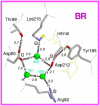
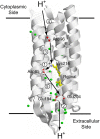
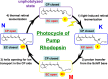
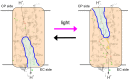
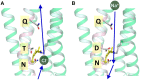
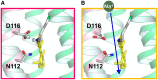
Rhodopsins are light-sensing proteins used in optogenetics. The word "rhodopsin" originates from the Greek words "rhodo" and "opsis," indicating rose and sight, respectively. Although the classical meaning of rhodopsin is the red-colored pigment in our eyes, the modern meaning of rhodopsin encompasses photoactive proteins containing a retinal chromophore in animals and microbes. Animal and microbial rhodopsins possess 11-cis and all-trans retinal, respectively, to capture light in seven transmembrane α-helices, and photoisomerizations into all-trans and 13-cis forms, respectively, initiate each function. Ion-transporting proteins can be found in microbial rhodopsins, such as light-gated channels and light-driven pumps, which are the main tools in optogenetics. Light-driven pumps, such as archaeal H(+) pump bacteriorhodopsin (BR) and Cl(-) pump halorhodopsin (HR), were discovered in the 1970s, and their mechanism has been extensively studied. On the other hand, different kinds of H(+) and Cl(-) pumps have been found in marine bacteria, such as proteorhodopsin (PR) and Fulvimarina pelagi rhodopsin (FR), respectively. In addition, a light-driven Na(+) pump was found, Krokinobacter eikastus rhodopsin 2 (KR2). These light-driven ion-pumping microbial rhodopsins are classified as DTD, TSA, DTE, NTQ, and NDQ rhodopsins for BR, HR, PR, FR, and KR2, respectively. Recent understanding of ion-pumping microbial rhodopsins is reviewed in this paper.
Keywords: H+ transfer; hydrogen bond; light-driven pump; photocycle; photoisomerizatoin; retinal; structural change.
Figures


Similar articles
-
History and Perspectives of Ion-Transporting Rhodopsins.Adv Exp Med Biol. 2021;1293:3-19. doi: 10.1007/978-981-15-8763-4_1. Adv Exp Med Biol. 2021. PMID: 33398804 Review.
-
Biophysics of rhodopsins and optogenetics.Biophys Rev. 2020 Apr;12(2):355-361. doi: 10.1007/s12551-020-00645-0. Epub 2020 Feb 17. Biophys Rev. 2020. PMID: 32065378 Free PMC article. Review.
-
Spectroscopic study of a light-driven chloride ion pump from marine bacteria.J Phys Chem B. 2014 Sep 25;118(38):11190-9. doi: 10.1021/jp507219q. Epub 2014 Sep 11. J Phys Chem B. 2014. PMID: 25166488
-
FTIR spectroscopy of a light-driven compatible sodium ion-proton pumping rhodopsin at 77 K.J Phys Chem B. 2014 May 8;118(18):4784-92. doi: 10.1021/jp500756f. Epub 2014 Apr 28. J Phys Chem B. 2014. PMID: 24773264
-
The light-driven sodium ion pump: A new player in rhodopsin research.Bioessays. 2016 Dec;38(12):1274-1282. doi: 10.1002/bies.201600065. Epub 2016 Nov 17. Bioessays. 2016. PMID: 27859420 Review.
Cited by
-
Protein dynamics of a light-driven Na+ pump rhodopsin probed using a tryptophan residue near the retinal chromophore.Biophys Physicobiol. 2023 Feb 25;20(Supplemental):e201016. doi: 10.2142/biophysico.bppb-v20.s016. eCollection 2023 Mar 21. Biophys Physicobiol. 2023. PMID: 38362331 Free PMC article.
-
Structural studies of bacteriorhodopsin in BC era.Biophys Physicobiol. 2023 Jan 19;20(Supplemental):e201006. doi: 10.2142/biophysico.bppb-v20.s006. eCollection 2023 Mar 21. Biophys Physicobiol. 2023. PMID: 38362329 Free PMC article.
-
Solid-state NMR for the characterization of retinal chromophore and Schiff base in TAT rhodopsin embedded in membranes under weakly acidic conditions.Biophys Physicobiol. 2023 Mar 2;20(Supplemental):e201017. doi: 10.2142/biophysico.bppb-v20.s017. eCollection 2023 Mar 21. Biophys Physicobiol. 2023. PMID: 38362323 Free PMC article.
-
Channel Gating in Kalium Channelrhodopsin Slow Mutants.J Mol Biol. 2024 Mar 1;436(5):168298. doi: 10.1016/j.jmb.2023.168298. Epub 2023 Oct 5. J Mol Biol. 2024. PMID: 37802216 Free PMC article.
-
A comparative study reveals the relative importance of prokaryotic and eukaryotic proton pump rhodopsins in a subtropical marginal sea.ISME Commun. 2023 Aug 18;3(1):79. doi: 10.1038/s43705-023-00292-y. ISME Commun. 2023. PMID: 37596487 Free PMC article.
References
-
- Agmon N. (1995). The Grotthuss mechanism. Chem. Phys. Lett. 244, 456–462. 10.1016/0009-2614(95)00905-J - DOI
-
- Balashov S. P., Petrovskaya L. E., Imasheva E. S., Lukashev E. P., Dioumaev A. K., Wang J. M., et al. . (2013). Breaking the carboxyl rule. Lysine96 facilitates protonation of the Schifff base in the photocycle of a retinal protein from Exiguobacterium sibiricum. J. Biol. Chem. 288, 21254–21265. 10.1074/jbc.M113.465138 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials


