A Molecular Approach Designed to Limit the Replication of Mature DENV2 in Host Cells
- PMID: 26154890
- PMCID: PMC4560859
- DOI: 10.1089/vim.2015.0034
A Molecular Approach Designed to Limit the Replication of Mature DENV2 in Host Cells
Abstract
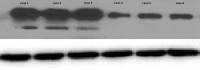
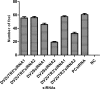
Dengue virus (DENV) is an arthropod-borne virus, which belongs to the Flaviviridae family, and completes its life cycle in two hosts: humans and mosquitoes. For DENV maturation, the surface pre-membrane (prM) protein is cleaved to form a mature membrane protein (M) by furin, which is a cellular enzyme subsequently releasing the mature virus from the host dendritic cell. The objective of the current study was to inhibit mature DENV isotype 2 (DENV2) by RNA-interference in a Vero-81 cell line. Mature DENV2 was propagated in and isolated from U937 cells expressing dendritic cell-specific intracellular adhesion molecule-3-grabbing non-integrin. Maturation of DENV2 was confirmed by Western blot analysis, where virus stock lacking prM was considered mature. Inhibition studies were carried out by transfection of Vero-81 cells with six synthetic siRNAs along with a control siRNA. Reduction in cellular DENV2 was observed also by focus-reduction assay, immunofluorescence assay (IFA), and real-time quantitative polymerase chain reaction (RT-qPCR). Cells transfected with DENV2SsiRNA2, which was targeting the structural region M of mature DENV2, was able to reduce DENV2 titer by up to 85% in focus reduction assays. A significant reduction in mature DENV2 RNA load was observed by RT-qPCR, confirming the previous findings. IFA also revealed reduced levels of cellular DENV2. These results demonstrated that mature DENV2 can be effectively inhibited by synthetic siRNA targeting the structural region of the genome. Mature DENV2 can be successfully inhibited by siRNAs, and specifically high knock-down efficiency is observed by siRNAs against M region of mature DENV2. This study shows that M represents a potential target for RNAi based inhibitory approaches.
Figures
Similar articles
-
[Study on the inhibitory effect of RNA interference on replication of dengue virus].Bing Du Xue Bao. 2010 Sep;26(5):373-8. Bing Du Xue Bao. 2010. PMID: 21043137 Chinese.
-
Inhibition of dengue virus infection by small interfering RNAs that target highly conserved sequences in the NS4B or NS5 coding regions.Arch Virol. 2018 May;163(5):1331-1335. doi: 10.1007/s00705-018-3757-2. Epub 2018 Feb 1. Arch Virol. 2018. PMID: 29392497
-
Tatanan A from the Acorus calamus L. root inhibited dengue virus proliferation and infections.Phytomedicine. 2018 Mar 15;42:258-267. doi: 10.1016/j.phymed.2018.03.018. Epub 2018 Mar 15. Phytomedicine. 2018. PMID: 29655694
-
RNA interference, arthropod-borne viruses, and mosquitoes.Virus Res. 2004 Jun 1;102(1):65-74. doi: 10.1016/j.virusres.2004.01.017. Virus Res. 2004. PMID: 15068882 Review.
-
RNAi: antiviral therapy against dengue virus.Asian Pac J Trop Biomed. 2013 Mar;3(3):232-6. doi: 10.1016/S2221-1691(13)60057-X. Asian Pac J Trop Biomed. 2013. PMID: 23620845 Free PMC article. Review.
References
-
- Birmingham A, Anderson E, Sullivan K, et al. . A protocol for designing siRNAs with high functionality and specificity. Nat Protoc 2007;2:2068–2078 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources


