Central nervous system regulation of brown adipose tissue
- PMID: 25428857
- PMCID: PMC4435534
- DOI: 10.1002/cphy.c140013
Central nervous system regulation of brown adipose tissue
Abstract
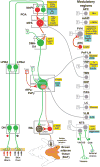
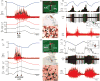
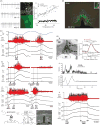
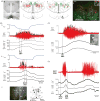
Thermogenesis, the production of heat energy, in brown adipose tissue is a significant component of the homeostatic repertoire to maintain body temperature during the challenge of low environmental temperature in many species from mouse to man and plays a key role in elevating body temperature during the febrile response to infection. The sympathetic neural outflow determining brown adipose tissue (BAT) thermogenesis is regulated by neural networks in the CNS which increase BAT sympathetic nerve activity in response to cutaneous and deep body thermoreceptor signals. Many behavioral states, including wakefulness, immunologic responses, and stress, are characterized by elevations in core body temperature to which central command-driven BAT activation makes a significant contribution. Since energy consumption during BAT thermogenesis involves oxidation of lipid and glucose fuel molecules, the CNS network driving cold-defensive and behavioral state-related BAT activation is strongly influenced by signals reflecting the short- and long-term availability of the fuel molecules essential for BAT metabolism and, in turn, the regulation of BAT thermogenesis in response to metabolic signals can contribute to energy balance, regulation of body adipose stores and glucose utilization. This review summarizes our understanding of the functional organization and neurochemical influences within the CNS networks that modulate the level of BAT sympathetic nerve activity to produce the thermoregulatory and metabolic alterations in BAT thermogenesis and BAT energy expenditure that contribute to overall energy homeostasis and the autonomic support of behavior.
Figures


Similar articles
-
Central neural regulation of brown adipose tissue thermogenesis and energy expenditure.Cell Metab. 2014 May 6;19(5):741-756. doi: 10.1016/j.cmet.2014.02.007. Epub 2014 Mar 13. Cell Metab. 2014. PMID: 24630813 Free PMC article. Review.
-
Central regulation of brown adipose tissue thermogenesis and energy homeostasis dependent on food availability.Pflugers Arch. 2018 May;470(5):823-837. doi: 10.1007/s00424-017-2090-z. Epub 2017 Dec 5. Pflugers Arch. 2018. PMID: 29209779 Free PMC article. Review.
-
Central control of brown adipose tissue thermogenesis.Front Endocrinol (Lausanne). 2012 Jan 24;3(5):5. doi: 10.3389/fendo.2012.00005. Front Endocrinol (Lausanne). 2012. PMID: 22389645 Free PMC article.
-
Thermogenesis in human brown adipose tissue and skeletal muscle induced by sympathomimetic stimulation.Acta Endocrinol Suppl (Copenh). 1986;278:1-32. Acta Endocrinol Suppl (Copenh). 1986. PMID: 3464154
-
Role of brown adipose tissue thermogenesis in control of thermoregulatory feeding in rats: a new hypothesis that links thermostatic and glucostatic hypotheses for control of food intake.Proc Soc Exp Biol Med. 1995 Feb;208(2):159-69. doi: 10.3181/00379727-208-43847a. Proc Soc Exp Biol Med. 1995. PMID: 7831348 Review.
Cited by
-
The Afferent Function of Adipose Innervation.Diabetes. 2024 Mar 1;73(3):348-354. doi: 10.2337/dbi23-0002. Diabetes. 2024. PMID: 38377447 Review.
-
A century of exercise physiology: concepts that ignited the study of human thermoregulation. Part 3: Heat and cold tolerance during exercise.Eur J Appl Physiol. 2024 Jan;124(1):1-145. doi: 10.1007/s00421-023-05276-3. Epub 2023 Oct 5. Eur J Appl Physiol. 2024. PMID: 37796292 Review.
-
Kappa opioid receptor activation increases thermogenic energy expenditure which drives increased feeding.iScience. 2023 Jun 28;26(7):107241. doi: 10.1016/j.isci.2023.107241. eCollection 2023 Jul 21. iScience. 2023. PMID: 37485355 Free PMC article.
-
Chronic social stress blunts core body temperature and molecular rhythms of Rbm3 and Cirbp in mouse lateral habenula.Open Biol. 2023 Jul;13(7):220380. doi: 10.1098/rsob.220380. Epub 2023 Jul 19. Open Biol. 2023. PMID: 37463657 Free PMC article.
-
Potential links between brown adipose tissue, circadian dysregulation, and suicide risk.Front Neurosci. 2023 Jun 9;17:1196029. doi: 10.3389/fnins.2023.1196029. eCollection 2023. Front Neurosci. 2023. PMID: 37360180 Free PMC article.
References
-
- Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, Garami A, Bautista D, Gavva NR, Romanovsky AA. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32:2086–2099. - PMC - PubMed
-
- Amini-Sereshki L, Zarrindast MR. Brain stem tonic inhibition of thermoregulation in the rat. Am J Physiol. 1984;247:R154–R159. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


