Effectiveness of the electronic cigarette: An eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints
- PMID: 25358095
- PMCID: PMC4245610
- DOI: 10.3390/ijerph111111220
Effectiveness of the electronic cigarette: An eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints
Abstract
Background: Smoking reduction remains a pivotal issue in public health policy, but quit rates obtained with traditional quit-smoking therapies remain disappointingly low. Tobacco Harm Reduction (THR), aiming at less harmful ways of consuming nicotine, may provide a more effective alternative. One promising candidate for THR are electronic cigarettes (e-cigs). The aim of this study was to investigate the efficacy of second-generation e-cigs both in terms of acute craving-reduction in the lab and in terms of smoking reduction and experienced benefits/complaints in an eight-month Randomized Controlled Trial (RCT).
Design: RCT with three arms.
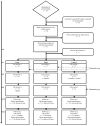
Methods: Participants (N = 48) unwilling to quit smoking were randomized into two e-cig groups and one control group. During three lab sessions (over two months) participants, who had been abstinent for four hours, vaped/smoked for five minutes, after which we monitored the effect on craving and withdrawal symptoms. eCO and saliva cotinine levels were also measured. In between lab sessions, participants in the e-cig groups could use e-cigs or smoke ad libitum, whereas the control group could only smoke. After the lab sessions, the control group also received an e-cig. The RCT included several questionnaires, which repeatedly monitored the effect of ad libitum e-cig use on the use of tobacco cigarettes and the experienced benefits/complaints up to six months after the last lab session.
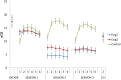
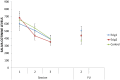
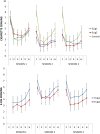
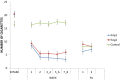
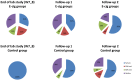
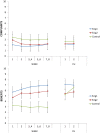
Results: From the first lab session on, e-cig use after four hours of abstinence resulted in a reduction in cigarette craving which was of the same magnitude as when a cigarette was smoked, while eCO was unaffected. After two months, we observed that 34% of the e-cig groups had stopped smoking tobacco cigarettes, versus 0% of the control group. After five months, the e-cig groups demonstrated a total quit-rate of 37%, whereas the control group showed a quit rate of 38% three months after initiating e-cig use. At the end of the eight-month study, 19% of the e-cig groups and 25% of the control group were totally abstinent from smoking, while an overall reduction of 60% in the number of cigarettes smoked per day was observed (compared to intake). eCO levels decreased, whereas cotinine levels were the same in all groups at each moment of measurement. Reported benefits far outweighed the reported complaints.
Conclusion: In a series of controlled lab sessions with e-cig naïve tobacco smokers, second generation e-cigs were shown to be immediately and highly effective in reducing abstinence induced cigarette craving and withdrawal symptoms, while not resulting in increases in eCO. Remarkable (>50 pc) eight-month reductions in, or complete abstinence from tobacco smoking was achieved with the e-cig in almost half (44%) of the participants.
Figures
Similar articles
-
Effects of nicotine versus placebo e-cigarette use on symptom relief during initial tobacco abstinence.Exp Clin Psychopharmacol. 2017 Aug;25(4):249-254. doi: 10.1037/pha0000134. Epub 2017 Jun 26. Exp Clin Psychopharmacol. 2017. PMID: 28650184 Free PMC article.
-
Successful Nicotine Intake in Medical Assisted Use of E-Cigarettes: A Pilot Study.Int J Environ Res Public Health. 2015 Jul 8;12(7):7638-46. doi: 10.3390/ijerph120707638. Int J Environ Res Public Health. 2015. PMID: 26184244 Free PMC article.
-
[Smoking reduction and temporary abstinence: new approaches for smoking cessation].J Mal Vasc. 2003 Dec;28(5):293-300. J Mal Vasc. 2003. PMID: 14978435 Review. French.
-
Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study.BMC Public Health. 2011 Oct 11;11:786. doi: 10.1186/1471-2458-11-786. BMC Public Health. 2011. PMID: 21989407 Free PMC article. Clinical Trial.
-
Electronic Cigarettes for Smoking Cessation: A Systematic Review.Nicotine Tob Res. 2016 Oct;18(10):1926-1936. doi: 10.1093/ntr/ntw119. Epub 2016 Apr 25. Nicotine Tob Res. 2016. PMID: 27113014 Review.
Cited by
-
The potential of new nicotine and tobacco products as tools for people who smoke to quit combustible cigarettes - a systematic review of common practices and guidance towards a robust study protocol to measure cessation efficacy.Harm Reduct J. 2024 Jul 5;21(1):130. doi: 10.1186/s12954-024-01047-1. Harm Reduct J. 2024. PMID: 38970058 Free PMC article.
-
New opportunities with ENDS for people who smoke and do not intend to quit smoking.Intern Emerg Med. 2024 Sep;19(6):1775-1777. doi: 10.1007/s11739-024-03677-6. Epub 2024 Jun 19. Intern Emerg Med. 2024. PMID: 38898216 No abstract available.
-
An exploratory, randomised, crossover study to investigate the effect of nicotine on cognitive function in healthy adult smokers who use an electronic cigarette after a period of smoking abstinence.Harm Reduct J. 2024 Apr 6;21(1):78. doi: 10.1186/s12954-024-00993-0. Harm Reduct J. 2024. PMID: 38582919 Free PMC article. Clinical Trial.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2024 Jan 8;1(1):CD010216. doi: 10.1002/14651858.CD010216.pub8. Cochrane Database Syst Rev. 2024. PMID: 38189560
-
A multicenter prospective randomized controlled trial investigating the effects of combustion-free nicotine alternatives on cardiovascular risk factors and metabolic parameters in individuals with type 2 diabetes who smoke: the DiaSmokeFree study protocol.Intern Emerg Med. 2024 Mar;19(2):321-332. doi: 10.1007/s11739-023-03467-6. Epub 2023 Nov 24. Intern Emerg Med. 2024. PMID: 37999870 Free PMC article.
References
-
- World Health Organization (WHO) WHO Report on the Global Tobacco Epidemic: Enforcing Bans on Tobacco Advertising, Promotion and Sponsorship. World Health Organization Press; Geneva, Switzerland: 2013.
-
- GfK Significant Rookgedrag. in België.: Een. Rapport aan Stichting tegen Kanker. [(accessed on 20 February 2014)]. Available online: www.kanker.be/sites/default/files/rookenquete_2013.pdf.
-
- TNS Opinion & Social Attitudes of Europeans towards Tobacco. [(accessed on 20 February 2014)]. Available online: http://ec.europa.eu/public_opinion/archives/ebs/ebs_385_en.pdf.
-
- World Health Organization (WHO) Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. World Health Organization Press; Geneva, Switzerland: 2009.
-
- World Health Organization (WHO) WHO Global Report: Mortality Attributable to Tobacco. World Health Organization Press; Geneva, Switzerland: 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous