Double-stranded RNA under force and torque: similarities to and striking differences from double-stranded DNA
- PMID: 25313077
- PMCID: PMC4217419
- DOI: 10.1073/pnas.1407197111
Double-stranded RNA under force and torque: similarities to and striking differences from double-stranded DNA
Abstract
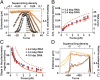
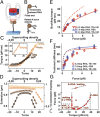
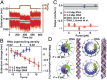
RNA plays myriad roles in the transmission and regulation of genetic information that are fundamentally constrained by its mechanical properties, including the elasticity and conformational transitions of the double-stranded (dsRNA) form. Although double-stranded DNA (dsDNA) mechanics have been dissected with exquisite precision, much less is known about dsRNA. Here we present a comprehensive characterization of dsRNA under external forces and torques using magnetic tweezers. We find that dsRNA has a force-torque phase diagram similar to that of dsDNA, including plectoneme formation, melting of the double helix induced by torque, a highly overwound state termed "P-RNA," and a highly underwound, left-handed state denoted "L-RNA." Beyond these similarities, our experiments reveal two unexpected behaviors of dsRNA: Unlike dsDNA, dsRNA shortens upon overwinding, and its characteristic transition rate at the plectonemic buckling transition is two orders of magnitude slower than for dsDNA. Our results challenge current models of nucleic acid mechanics, provide a baseline for modeling RNAs in biological contexts, and pave the way for new classes of magnetic tweezers experiments to dissect the role of twist and torque for RNA-protein interactions at the single-molecule level.
Keywords: RNA; force; magnetic tweezers; nucleic acids; torque.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Blind predictions of DNA and RNA tweezers experiments with force and torque.PLoS Comput Biol. 2014 Aug 7;10(8):e1003756. doi: 10.1371/journal.pcbi.1003756. eCollection 2014 Aug. PLoS Comput Biol. 2014. PMID: 25102226 Free PMC article.
-
Understanding the mechanical response of double-stranded DNA and RNA under constant stretching forces using all-atom molecular dynamics.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):7049-7054. doi: 10.1073/pnas.1705642114. Epub 2017 Jun 20. Proc Natl Acad Sci U S A. 2017. PMID: 28634300 Free PMC article.
-
Probing the mechanical properties, conformational changes, and interactions of nucleic acids with magnetic tweezers.J Struct Biol. 2017 Jan;197(1):26-36. doi: 10.1016/j.jsb.2016.06.022. Epub 2016 Jun 29. J Struct Biol. 2017. PMID: 27368129 Review.
-
Explaining the striking difference in twist-stretch coupling between DNA and RNA: A comparative molecular dynamics analysis.Nucleic Acids Res. 2015 Dec 2;43(21):10143-56. doi: 10.1093/nar/gkv1028. Epub 2015 Oct 12. Nucleic Acids Res. 2015. PMID: 26464435 Free PMC article.
-
Mechanisms and applications of peptide nucleic acids selectively binding to double-stranded RNA.Biopolymers. 2022 Feb;113(2):e23476. doi: 10.1002/bip.23476. Epub 2021 Sep 28. Biopolymers. 2022. PMID: 34581432 Review.
Cited by
-
Effect of temperature on anisotropic bending elasticity of dsRNA: an all-atom molecular dynamics simulation.RSC Adv. 2024 May 28;14(24):17170-17177. doi: 10.1039/d4ra02354d. eCollection 2024 May 22. RSC Adv. 2024. PMID: 38808231 Free PMC article.
-
Systematic Comparison of Atomistic Force Fields for the Mechanical Properties of Double-Stranded DNA.J Chem Theory Comput. 2024 Mar 12;20(5):2261-2272. doi: 10.1021/acs.jctc.3c01089. Epub 2024 Feb 27. J Chem Theory Comput. 2024. PMID: 38411091 Free PMC article.
-
Liquid crystalline inverted lipid phases encapsulating siRNA enhance lipid nanoparticle mediated transfection.Nat Commun. 2024 Feb 12;15(1):1303. doi: 10.1038/s41467-024-45666-5. Nat Commun. 2024. PMID: 38347001 Free PMC article.
-
Temperature-dependent elasticity of DNA, RNA, and hybrid double helices.Biophys J. 2024 Mar 5;123(5):572-583. doi: 10.1016/j.bpj.2024.01.032. Epub 2024 Feb 2. Biophys J. 2024. PMID: 38340722
-
The origin of different bending stiffness between double-stranded RNA and DNA revealed by magnetic tweezers and simulations.Nucleic Acids Res. 2024 Mar 21;52(5):2519-2529. doi: 10.1093/nar/gkae063. Nucleic Acids Res. 2024. PMID: 38321947 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


