Synthesis, properties, and biological activity of boranophosphate analogs of the mRNA cap: versatile tools for manipulation of therapeutically relevant cap-dependent processes
- PMID: 25150148
- PMCID: PMC4176373
- DOI: 10.1093/nar/gku757
Synthesis, properties, and biological activity of boranophosphate analogs of the mRNA cap: versatile tools for manipulation of therapeutically relevant cap-dependent processes
Abstract
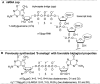
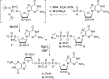
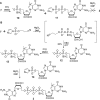
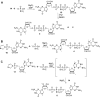
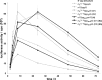
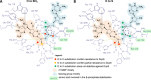
Modified mRNA cap analogs aid in the study of mRNA-related processes and may enable creation of novel therapeutic interventions. We report the synthesis and properties of 11 dinucleotide cap analogs bearing a single boranophosphate modification at either the α-, β- or γ-position of the 5',5'-triphosphate chain. The compounds can potentially serve either as inhibitors of translation in cancer cells or reagents for increasing expression of therapeutic proteins in vivo from exogenous mRNAs. The BH3-analogs were tested as substrates and binding partners for two major cytoplasmic cap-binding proteins, DcpS, a decapping pyrophosphatase, and eIF4E, a translation initiation factor. The susceptibility to DcpS was different between BH3-analogs and the corresponding analogs containing S instead of BH3 (S-analogs). Depending on its placement, the boranophosphate group weakened the interaction with DcpS but stabilized the interaction with eIF4E. The first of the properties makes the BH3-analogs more stable and the second, more potent as inhibitors of protein biosynthesis. Protein expression in dendritic cells was 2.2- and 1.7-fold higher for mRNAs capped with m2 (7,2'-O)GppBH3pG D1 and m2 (7,2'-O)GppBH3pG D2, respectively, than for in vitro transcribed mRNA capped with m2 (7,3'-O)GpppG. Higher expression of cancer antigens would make mRNAs containing m2 (7,2'-O)GppBH3pG D1 and m2 (7,2'-O)GppBH3pG D2 favorable for anticancer immunization.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Synthesis and characterization of mRNA cap analogs containing phosphorothioate substitutions that bind tightly to eIF4E and are resistant to the decapping pyrophosphatase DcpS.RNA. 2008 Jun;14(6):1119-31. doi: 10.1261/rna.990208. Epub 2008 Apr 22. RNA. 2008. PMID: 18430890 Free PMC article.
-
The first examples of mRNA cap analogs bearing boranophosphate modification.Nucleic Acids Symp Ser (Oxf). 2008;(52):289-90. doi: 10.1093/nass/nrn146. Nucleic Acids Symp Ser (Oxf). 2008. PMID: 18776367
-
Phosphorothioate analogs of m7GTP are enzymatically stable inhibitors of cap-dependent translation.Bioorg Med Chem Lett. 2009 Apr 1;19(7):1921-5. doi: 10.1016/j.bmcl.2009.02.053. Epub 2009 Feb 21. Bioorg Med Chem Lett. 2009. PMID: 19269171
-
Synthesis of anti-reverse cap analogs (ARCAs) and their applications in mRNA translation and stability.Methods Enzymol. 2007;431:203-27. doi: 10.1016/S0076-6879(07)31011-2. Methods Enzymol. 2007. PMID: 17923237 Review.
-
Potential therapeutic applications of RNA cap analogs.Future Med Chem. 2013 Jun;5(10):1141-72. doi: 10.4155/fmc.13.96. Future Med Chem. 2013. PMID: 23795970 Review.
Cited by
-
Application of Mammalian Nudix Enzymes to Capped RNA Analysis.Pharmaceuticals (Basel). 2024 Sep 11;17(9):1195. doi: 10.3390/ph17091195. Pharmaceuticals (Basel). 2024. PMID: 39338357 Free PMC article. Review.
-
Trinucleotide cap analogs with triphosphate chain modifications: synthesis, properties, and evaluation as mRNA capping reagents.Nucleic Acids Res. 2024 Oct 14;52(18):10788-10809. doi: 10.1093/nar/gkae763. Nucleic Acids Res. 2024. PMID: 39248095 Free PMC article.
-
Advances in non-viral mRNA delivery to the spleen.Biomater Sci. 2024 Jun 11;12(12):3027-3044. doi: 10.1039/d4bm00038b. Biomater Sci. 2024. PMID: 38712531 Review.
-
Advances in Nonviral mRNA Delivery Materials and Their Application as Vaccines for Melanoma Therapy.ACS Appl Bio Mater. 2024 Aug 19;7(8):4894-4913. doi: 10.1021/acsabm.3c00721. Epub 2023 Nov 6. ACS Appl Bio Mater. 2024. PMID: 37930174 Free PMC article. Review.
-
Chemical Modifications of mRNA Ends for Therapeutic Applications.Acc Chem Res. 2023 Oct 17;56(20):2814-2826. doi: 10.1021/acs.accounts.3c00442. Epub 2023 Oct 2. Acc Chem Res. 2023. PMID: 37782471 Free PMC article.
References
-
- Coller J., Parker R. Eukaryotic mRNA decapping. Annu. Rev. Biochem. 2004;73:861–890. - PubMed
-
- Sonenberg N. eIF4E, the mRNA cap-binding protein: from basic discovery to translational research. Biochem. Cell Biol. 2008;86:178–183. - PubMed
-
- Cougot N., van Dijk E., Babajko S., Séraphin B. ‘Cap-tabolism’. Trends Biochem. Sci. 2004;29:436–444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


