JNK is a novel regulator of intercellular adhesion
- PMID: 24868495
- PMCID: PMC3942331
- DOI: 10.4161/tisb.26845
JNK is a novel regulator of intercellular adhesion
Abstract
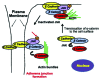
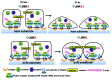
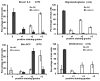
c-Jun N-terminal Kinase (JNK) is a family of protein kinases, which are activated by stress stimuli such as inflammation, heat stress and osmotic stress, and regulate diverse cellular processes including proliferation, survival and apoptosis. In this review, we focus on a recently discovered function of JNK as a regulator of intercellular adhesion. We summarize the existing knowledge regarding the role of JNK during the formation of cell-cell junctions. The potential mechanisms and implications for processes requiring dynamic formation and dissolution of cell-cell junctions including wound healing, migration, cancer metastasis and stem cell differentiation are also discussed.
Keywords: JNK; adherens junction; cell-cell adhesion; intercellular signaling; substrate stiffness.
Figures
Similar articles
-
RhoA-JNK Regulates the E-Cadherin Junctions of Human Gingival Epithelial Cells.J Dent Res. 2016 Mar;95(3):284-91. doi: 10.1177/0022034515619375. Epub 2015 Dec 3. J Dent Res. 2016. PMID: 26635280
-
c-Jun N-terminal kinase mediates disassembly of apical junctions in model intestinal epithelia.Cell Cycle. 2009 Jul 1;8(13):2110-21. doi: 10.4161/cc.8.13.8928. Epub 2009 Jul 5. Cell Cycle. 2009. PMID: 19502798
-
JNK regulates compliance-induced adherens junctions formation in epithelial cells and tissues.J Cell Sci. 2013 Jun 15;126(Pt 12):2718-29. doi: 10.1242/jcs.122903. Epub 2013 Apr 16. J Cell Sci. 2013. PMID: 23591817
-
The role of JNK in prostate cancer progression and therapeutic strategies.Biomed Pharmacother. 2020 Jan;121:109679. doi: 10.1016/j.biopha.2019.109679. Epub 2019 Nov 24. Biomed Pharmacother. 2020. PMID: 31810118 Review.
-
Abl tyrosine kinases modulate cadherin-dependent adhesion upstream and downstream of Rho family GTPases.Cell Cycle. 2008 Feb 15;7(4):444-8. doi: 10.4161/cc.7.4.5452. Epub 2007 Dec 12. Cell Cycle. 2008. PMID: 18235247 Review.
Cited by
-
Proteomic changes of the bovine blood plasma in response to heat stress in a tropically adapted cattle breed.Front Genet. 2024 Aug 1;15:1392670. doi: 10.3389/fgene.2024.1392670. eCollection 2024. Front Genet. 2024. PMID: 39149588 Free PMC article.
-
Crosstalk of MAP3K1 and EGFR signaling mediates gene-environment interactions that block developmental tissue closure.J Biol Chem. 2024 Jul;300(7):107486. doi: 10.1016/j.jbc.2024.107486. Epub 2024 Jun 18. J Biol Chem. 2024. PMID: 38897570 Free PMC article.
-
LSR antibody promotes apoptosis and disrupts epithelial barriers via signal pathways in endometrial cancer.Tissue Barriers. 2023 Jul 3;11(3):2106113. doi: 10.1080/21688370.2022.2106113. Epub 2022 Jul 26. Tissue Barriers. 2023. PMID: 35883247 Free PMC article.
-
Hydrogen Sulfide Inhibits Bronchial Epithelial Cell Epithelial Mesenchymal Transition Through Regulating Endoplasm Reticulum Stress.Front Mol Biosci. 2022 Apr 12;9:828766. doi: 10.3389/fmolb.2022.828766. eCollection 2022. Front Mol Biosci. 2022. PMID: 35495633 Free PMC article.
-
Cytoskeleton and Associated Proteins: Pleiotropic JNK Substrates and Regulators.Int J Mol Sci. 2021 Aug 4;22(16):8375. doi: 10.3390/ijms22168375. Int J Mol Sci. 2021. PMID: 34445080 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

