A randomized trial of concurrent smoking-cessation and substance use disorder treatment in stimulant-dependent smokers
- PMID: 24345356
- PMCID: PMC4019678
- DOI: 10.4088/JCP.13m08449
A randomized trial of concurrent smoking-cessation and substance use disorder treatment in stimulant-dependent smokers
Abstract
Objective: To evaluate the impact of concurrent treatments for substance use disorder and nicotine-dependence for stimulant-dependent patients.
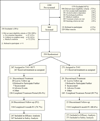
Method: A randomized, 10-week trial with follow-up at 3 and 6 months after smoking quit date conducted at 12 substance use disorder treatment programs between February 2010 and July 2012. Adults meeting DSM-IV-TR criteria for cocaine and/or methamphetamine dependence and interested in quitting smoking were randomized to treatment as usual (n = 271) or treatment as usual with smoking-cessation treatment (n = 267). All participants received treatment as usual for substance use disorder treatment. Participants assigned to treatment as usual with concurrent smoking-cessation treatment received weekly individual smoking cessation counseling and extended-release bupropion (300 mg/d) during weeks 1-10. During post-quit treatment (weeks 4-10), participants assigned to treatment as usual with smoking-cessation treatment received a nicotine inhaler and contingency management for smoking abstinence. Weekly proportion of stimulant-abstinent participants during the treatment phase, as assessed by urine drug screens and self-report, was the primary outcome. Secondary measures included other substance/nicotine use outcomes and treatment attendance.
Results: There were no significant treatment effects on stimulant-use outcomes, as measured by the primary outcome and stimulant-free days, on drug-abstinence, or on attendance. Participants assigned to treatment as usual with smoking-cessation treatment, relative to those assigned to treatment as usual, had significantly better outcomes for drug-free days at 6-month follow-up (χ(2)(1) = 4.09, P <.05), with a decrease in drug-free days from baseline of -1.3% in treatment as usual with smoking-cessation treatment and of -7.6% in treatment as usual. Participants receiving treatment as usual with smoking-cessation treatment, relative to those receiving treatment as usual, had significantly better outcomes on smoking point-prevalence abstinence (25.5% vs 2.2%; χ(2)(1) = 44.69, P < .001; OR =18.2).
Conclusions: These results suggest that providing smoking-cessation treatment to illicit stimulant-dependent patients in outpatient substance use disorder treatment will not worsen, and may enhance, abstinence from nonnicotine substance use.
Trial registration: ClinicalTrials.gov identifier: NCT01077024.
© Copyright 2013 Physicians Postgraduate Press, Inc.
Figures
Similar articles
-
Achieving smoking abstinence is associated with decreased cocaine use in cocaine-dependent patients receiving smoking-cessation treatment.Drug Alcohol Depend. 2014 Jan 1;134:391-395. doi: 10.1016/j.drugalcdep.2013.09.019. Epub 2013 Sep 27. Drug Alcohol Depend. 2014. PMID: 24128381 Free PMC article. Clinical Trial.
-
Design considerations for a study to evaluate the impact of smoking cessation treatment on stimulant use outcomes in stimulant-dependent individuals.Contemp Clin Trials. 2012 Jan;33(1):197-205. doi: 10.1016/j.cct.2011.09.018. Epub 2011 Oct 8. Contemp Clin Trials. 2012. PMID: 22005174 Free PMC article. Clinical Trial.
-
Evaluating Nicotine Craving, Withdrawal, and Substance Use as Mediators of Smoking Cessation in Cocaine- and Methamphetamine-Dependent Patients.Nicotine Tob Res. 2016 May;18(5):1196-201. doi: 10.1093/ntr/ntv121. Epub 2015 Jun 5. Nicotine Tob Res. 2016. PMID: 26048168 Free PMC article. Clinical Trial.
-
Smoking cessation interventions for smokers with current or past depression.Cochrane Database Syst Rev. 2013 Aug 21;(8):CD006102. doi: 10.1002/14651858.CD006102.pub2. Cochrane Database Syst Rev. 2013. PMID: 23963776 Review.
-
Nicotine receptor partial agonists for smoking cessation.Cochrane Database Syst Rev. 2012 Apr 18;(4):CD006103. doi: 10.1002/14651858.CD006103.pub6. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 May 09;(5):CD006103. doi: 10.1002/14651858.CD006103.pub7. PMID: 22513936 Updated. Review.
Cited by
-
Predictors of urine toxicology and other biologic specimen missingness in randomized trials of substance use disorders.Drug Alcohol Depend. 2024 Aug 1;261:111368. doi: 10.1016/j.drugalcdep.2024.111368. Epub 2024 Jun 12. Drug Alcohol Depend. 2024. PMID: 38896944
-
The ASAM/AAAP Clinical Practice Guideline on the Management of Stimulant Use Disorder.J Addict Med. 2024 May-Jun 01;18(1S Suppl 1):1-56. doi: 10.1097/ADM.0000000000001299. J Addict Med. 2024. PMID: 38669101
-
Potential effect of antidepressants on remission from cocaine use disorder - A nationwide matched retrospective cohort study.Drug Alcohol Depend. 2023 Oct 1;251:110958. doi: 10.1016/j.drugalcdep.2023.110958. Epub 2023 Sep 7. Drug Alcohol Depend. 2023. PMID: 37703770 Free PMC article.
-
A qualitative study on people with opioid use disorders' perspectives on smoking and smoking cessation interventions.Front Psychiatry. 2023 Aug 10;14:1185338. doi: 10.3389/fpsyt.2023.1185338. eCollection 2023. Front Psychiatry. 2023. PMID: 37636821 Free PMC article.
-
Naltrexone plus bupropion reduces cigarette smoking in individuals with methamphetamine use disorder: A secondary analysis from the CTN ADAPT-2 trial.J Subst Use Addict Treat. 2023 Aug;151:208987. doi: 10.1016/j.josat.2023.208987. Epub 2023 Feb 21. J Subst Use Addict Treat. 2023. PMID: 36822269 Free PMC article. Clinical Trial.
References
-
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226–1228. - PubMed
-
- Schroeder SA. A 51-year-old woman with bipolar disorder who wants to quit smoking. JAMA. 2009;301(5):522–531. - PubMed
-
- Hurt RD, Offord KP, Croghan IT, et al. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275(14):1097–1103. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 DA020024/DA/NIDA NIH HHS/United States
- U10-DA013045/DA/NIDA NIH HHS/United States
- U10 DA020036/DA/NIDA NIH HHS/United States
- U10 DA020024/DA/NIDA NIH HHS/United States
- U10-DA013727/DA/NIDA NIH HHS/United States
- U10 DA013045/DA/NIDA NIH HHS/United States
- U10 DA013727/DA/NIDA NIH HHS/United States
- U10 DA013720/DA/NIDA NIH HHS/United States
- U10-DA015815/DA/NIDA NIH HHS/United States
- U10-DA013720/DA/NIDA NIH HHS/United States
- U10-DA020024/DA/NIDA NIH HHS/United States
- U10-DA020036/DA/NIDA NIH HHS/United States
- U10 DA013732/DA/NIDA NIH HHS/United States
- U10-DA013732/DA/NIDA NIH HHS/United States
- U10 DA015815/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical