Enhanced receptor-mediated endocytosis and cytotoxicity of a folic acid-desacetylvinblastine monohydrazide conjugate in a pemetrexed-resistant cell line lacking folate-specific facilitative carriers but with increased folate receptor expression
- PMID: 24249723
- PMCID: PMC3913358
- DOI: 10.1124/mol.113.089110
Enhanced receptor-mediated endocytosis and cytotoxicity of a folic acid-desacetylvinblastine monohydrazide conjugate in a pemetrexed-resistant cell line lacking folate-specific facilitative carriers but with increased folate receptor expression
Abstract
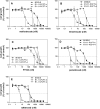
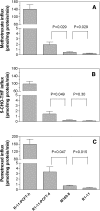
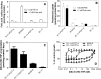
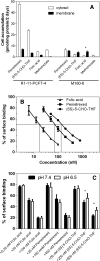
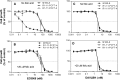
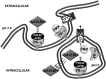
The reduced folate carrier (RFC), proton-coupled folate transporter (PCFT), and folate receptors (FR) are folate-specific transporters. Antifolates currently in the clinic, such as pemetrexed, methotrexate, and pralatrexate, are transported into tumor cells primarily via RFC. Folic acid conjugated to cytotoxics, a new class of antineoplastics, are transported into cells via FR-mediated endocytosis. To better define the role of PCFT in antifolate resistance, a methotrexate-resistant cell line, M160-8, was selected from a HeLa subline in which the RFC gene was deleted and PCFT was highly overexpressed. These cells were cross-resistant to pemetrexed. PCFT function and the PCFT mRNA level in M160-8 cells were barely detectable, and FR-α function and mRNA level were increased as compared with the parent cells. While pemetrexed rapidly associated with FR and was internalized within endosomes in M160-8 cells, consistent with FR-mediated transport, subsequent pemetrexed and (6S)-5-formyltetrahydrofolate export into the cytosol was markedly impaired. In contrast, M160-8 cells were collaterally sensitive to EC0905, a folic acid-desacetylvinblastine monohydrazide conjugate also transported by FR-mediated endocytosis. However, in this case a sulfhydryl bond is cleaved to release the lipophilic cytotoxic moiety into the endosome, which passively diffuses out of the endosome into the cytosol. Hence, resistance to pemetrexed in M160-8 cells was due to entrapment of the drug within the endosome due to the absence of PCFT under conditions in which the FR cycling function was intact.
Figures

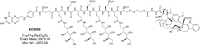
Similar articles
-
Determinants of the activities of antifolates delivered into cells by folate-receptor-mediated endocytosis.Cancer Chemother Pharmacol. 2015 Jun;75(6):1163-73. doi: 10.1007/s00280-015-2733-8. Epub 2015 Apr 7. Cancer Chemother Pharmacol. 2015. PMID: 25847479 Free PMC article.
-
Functional Characterization of Reduced Folate Carrier and Protein-Coupled Folate Transporter for Antifolates Accumulation in Non-Small Cell Lung Cancer Cells.Drug Metab Dispos. 2024 Oct 16;52(11):1332-1344. doi: 10.1124/dmd.124.001872. Drug Metab Dispos. 2024. PMID: 39261014
-
The proton-coupled folate transporter: impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier.Mol Pharmacol. 2008 Sep;74(3):854-62. doi: 10.1124/mol.108.045443. Epub 2008 Jun 4. Mol Pharmacol. 2008. PMID: 18524888 Free PMC article.
-
The major facilitative folate transporters solute carrier 19A1 and solute carrier 46A1: biology and role in antifolate chemotherapy of cancer.Drug Metab Dispos. 2014 Apr;42(4):632-49. doi: 10.1124/dmd.113.055723. Epub 2014 Jan 6. Drug Metab Dispos. 2014. PMID: 24396145 Free PMC article. Review.
-
Biology of the major facilitative folate transporters SLC19A1 and SLC46A1.Curr Top Membr. 2014;73:175-204. doi: 10.1016/B978-0-12-800223-0.00004-9. Curr Top Membr. 2014. PMID: 24745983 Free PMC article. Review.
Cited by
-
PEGylation and folic-acid functionalization of cationic lipoplexes-Improved nucleic acid transfer into cancer cells.Front Bioeng Biotechnol. 2022 Dec 21;10:1066887. doi: 10.3389/fbioe.2022.1066887. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36619382 Free PMC article.
-
Folate-appended cyclodextrin carrier targets ovarian cancer cells expressing the proton-coupled folate transporter.Cancer Sci. 2020 May;111(5):1794-1804. doi: 10.1111/cas.14379. Epub 2020 Apr 3. Cancer Sci. 2020. PMID: 32154964 Free PMC article.
-
PBPK modeling-based optimization of site-specific chemo-photodynamic therapy with far-red light-activatable paclitaxel prodrug.J Control Release. 2019 Aug 28;308:86-97. doi: 10.1016/j.jconrel.2019.07.010. Epub 2019 Jul 9. J Control Release. 2019. PMID: 31299262 Free PMC article.
-
Microfluidic Synthesis of Vinblastine-Loaded Multifunctional Particles for Magnetically Responsive Controlled Drug Release.Pharmaceutics. 2019 May 3;11(5):212. doi: 10.3390/pharmaceutics11050212. Pharmaceutics. 2019. PMID: 31058849 Free PMC article.
-
Concentrative Transport of Antifolates Mediated by the Proton-Coupled Folate Transporter (SLC46A1); Augmentation by a HEPES Buffer.Mol Pharmacol. 2018 Mar;93(3):208-215. doi: 10.1124/mol.117.110445. Epub 2018 Jan 11. Mol Pharmacol. 2018. PMID: 29326243 Free PMC article.
References
-
- Ashokkumar B, Mohammed ZM, Vaziri ND, Said HM. (2007) Effect of folate oversupplementation on folate uptake by human intestinal and renal epithelial cells. Am J Clin Nutr 86:159–166 - PubMed
-
- Brigle KE, Spinella MJ, Westin EH, Goldman ID. (1994) Increased expression and characterization of two distinct folate binding proteins in murine erythroleukemia cells. Biochem Pharmacol 47:337–345 - PubMed
-
- Cario H, Bode H, Debatin KM, Opladen T, Schwarz K. (2009) Congenital null mutations of the FOLR1 gene: a progressive neurologic disease and its treatment. Neurology 73:2127–2129 - PubMed
-
- Chattopadhyay S, Zhao R, Krupenko SA, Krupenko N, Goldman ID. (2006) The inverse relationship between reduced folate carrier function and pemetrexed activity in a human colon cancer cell line. Mol Cancer Ther 5:438–449 - PubMed
-
- Desmoulin SK, Wang L, Polin L, White K, Kushner J, Stout M, Hou Z, Cherian C, Gangjee A, Matherly LH. (2012) Functional loss of the reduced folate carrier enhances the antitumor activities of novel antifolates with selective uptake by the proton-coupled folate transporter. Mol Pharmacol 82:591–600 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

