NMDA receptor activation and calpain contribute to disruption of dendritic spines by the stress neuropeptide CRH
- PMID: 24155300
- PMCID: PMC3807024
- DOI: 10.1523/JNEUROSCI.1445-13.2013
NMDA receptor activation and calpain contribute to disruption of dendritic spines by the stress neuropeptide CRH
Abstract
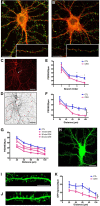
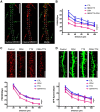
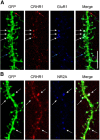
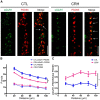
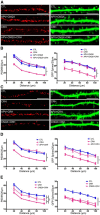
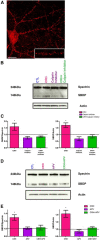
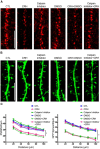
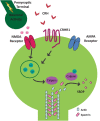
The complex effects of stress on learning and memory are mediated, in part, by stress-induced changes in the composition and structure of excitatory synapses. In the hippocampus, the effects of stress involve several factors including glucocorticoids and the stress-released neuropeptide corticotropin-releasing hormone (CRH), which influence the integrity of dendritic spines and the structure and function of the excitatory synapses they carry. CRH, at nanomolar, presumed-stress levels, rapidly abolishes short-term synaptic plasticity and destroys dendritic spines, yet the mechanisms for these effects are not fully understood. Here we tested the hypothesis that glutamate receptor-mediated processes, which shape synaptic structure and function, are engaged by CRH and contribute to spine destabilization. In cultured rat hippocampal neurons, CRH application reduced dendritic spine density in a time- and dose-dependent manner, and this action depended on the CRH receptor type 1. CRH-mediated spine loss required network activity and the activation of NMDA, but not of AMPA receptors; indeed GluR1-containing dendritic spines were resistant to CRH. Downstream of NMDA receptors, the calcium-dependent enzyme, calpain, was recruited, resulting in the breakdown of spine actin-interacting proteins including spectrin. Pharmacological approaches demonstrated that calpain recruitment contributed critically to CRH-induced spine loss. In conclusion, the stress hormone CRH co-opts mechanisms that contribute to the plasticity and integrity of excitatory synapses, leading to selective loss of dendritic spines. This spine loss might function as an adaptive mechanism preventing the consequences of adverse memories associated with severe stress.
Figures
Similar articles
-
Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone.J Neurosci. 2008 Mar 12;28(11):2903-11. doi: 10.1523/JNEUROSCI.0225-08.2008. J Neurosci. 2008. PMID: 18337421 Free PMC article.
-
Corticotropin-releasing hormone (CRH) depresses n-methyl-D-aspartate receptor-mediated current in cultured rat hippocampal neurons via CRH receptor type 1.Endocrinology. 2008 Mar;149(3):1389-98. doi: 10.1210/en.2007-1378. Epub 2007 Dec 13. Endocrinology. 2008. PMID: 18079206
-
Non-Ionotropic NMDA Receptor Signaling Drives Activity-Induced Dendritic Spine Shrinkage.J Neurosci. 2015 Sep 2;35(35):12303-8. doi: 10.1523/JNEUROSCI.4289-14.2015. J Neurosci. 2015. PMID: 26338340 Free PMC article.
-
Stress and anxiety in schizophrenia and depression: glucocorticoids, corticotropin-releasing hormone and synapse regression.Aust N Z J Psychiatry. 2008 Dec;42(12):995-1002. doi: 10.1080/00048670802512073. Aust N Z J Psychiatry. 2008. PMID: 19016087 Review.
-
Regulation of neuronal PKA signaling through AKAP targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
Cited by
-
Enduring memory consequences of early-life stress / adversity: Structural, synaptic, molecular and epigenetic mechanisms.Neurobiol Stress. 2024 Aug 30;33:100669. doi: 10.1016/j.ynstr.2024.100669. eCollection 2024 Nov. Neurobiol Stress. 2024. PMID: 39309367 Free PMC article. Review.
-
Cisplatin induces BDNF downregulation in middle-aged female rat model while BDNF enhancement attenuates cisplatin neurotoxicity.Exp Neurol. 2024 May;375:114717. doi: 10.1016/j.expneurol.2024.114717. Epub 2024 Feb 8. Exp Neurol. 2024. PMID: 38336286 Free PMC article.
-
Introduction: What Are Dendritic Spines?Adv Neurobiol. 2023;34:1-68. doi: 10.1007/978-3-031-36159-3_1. Adv Neurobiol. 2023. PMID: 37962793
-
Genes positively regulated by Mef2c in cortical neurons are enriched for common genetic variation associated with IQ and educational attainment.Hum Mol Genet. 2023 Nov 3;32(22):3194-3203. doi: 10.1093/hmg/ddad142. Hum Mol Genet. 2023. PMID: 37672226 Free PMC article.
-
The Yin and Yang of GABAergic and Glutamatergic Synaptic Plasticity: Opposites in Balance by Crosstalking Mechanisms.Front Synaptic Neurosci. 2022 May 19;14:911020. doi: 10.3389/fnsyn.2022.911020. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35663370 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

