Genetically-defined metabolic reprogramming in cancer
- PMID: 22858391
- PMCID: PMC3466334
- DOI: 10.1016/j.tem.2012.06.009
Genetically-defined metabolic reprogramming in cancer
Abstract
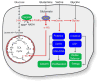
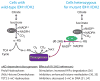
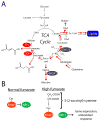
Oncogenes and tumor suppressors regulate cell metabolism. Evidence demonstrates that tumorigenic mutations in these genes tend to orchestrate metabolic activity into a platform that promotes cell survival, growth, and proliferation. Recent work has shown that some metabolic enzymes are also mutated in cancer, and that these mutations may influence malignancy directly. Thus, these enzymes seem to function as oncogenes and tumor suppressors, and would appear to be compelling targets for therapeutic intervention. Here, we review several enzymes mutated in cancer - phosphoglycerate dehydrogenase, isocitrate dehydrogenases 1 and 2, succinate dehydrogenase, and fumarate hydratase - and discuss exciting new work that has begun to pull back the curtain on how mutations in these enzymes influence tumorigenesis.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Isocitrate dehydrogenase (IDH), succinate dehydrogenase (SDH), fumarate hydratase (FH): three players for one phenotype in cancer?Biochem Soc Trans. 2016 Aug 15;44(4):1111-6. doi: 10.1042/BST20160099. Biochem Soc Trans. 2016. PMID: 27528759 Review.
-
Metabolic enzymes as oncogenes or tumor suppressors.N Engl J Med. 2009 Feb 19;360(8):813-5. doi: 10.1056/NEJMe0810213. N Engl J Med. 2009. PMID: 19228626 Free PMC article. No abstract available.
-
Revisiting the TCA cycle: signaling to tumor formation.Trends Mol Med. 2011 Nov;17(11):641-9. doi: 10.1016/j.molmed.2011.06.001. Epub 2011 Jul 20. Trends Mol Med. 2011. PMID: 21764377 Free PMC article. Review.
-
Metabolic alteration in tumorigenesis.Sci China Life Sci. 2013 Dec;56(12):1067-75. doi: 10.1007/s11427-013-4549-2. Epub 2013 Oct 10. Sci China Life Sci. 2013. PMID: 24114443 Review.
-
Metabolic reprogramming and epithelial-to-mesenchymal transition in cancer.FEBS J. 2017 Oct;284(19):3132-3144. doi: 10.1111/febs.14090. Epub 2017 May 21. FEBS J. 2017. PMID: 28444969 Free PMC article. Review.
Cited by
-
Preclinical efficacy of CBR-5884 against epithelial ovarian cancer cells by targeting the serine synthesis pathway.Discov Oncol. 2024 May 11;15(1):154. doi: 10.1007/s12672-024-01013-0. Discov Oncol. 2024. PMID: 38733440 Free PMC article.
-
PHGDH is Key to a Prognostic Multigene Signature and a Potential Therapeutic Target in Acute Myeloid Leukemia.J Cancer. 2024 Mar 11;15(9):2538-2548. doi: 10.7150/jca.90822. eCollection 2024. J Cancer. 2024. PMID: 38577610 Free PMC article.
-
Succinate metabolism: a promising therapeutic target for inflammation, ischemia/reperfusion injury and cancer.Front Cell Dev Biol. 2023 Sep 22;11:1266973. doi: 10.3389/fcell.2023.1266973. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37808079 Free PMC article. Review.
-
The impact of RNA binding proteins and the associated long non-coding RNAs in the TCA cycle on cancer pathogenesis.RNA Biol. 2023 Jan;20(1):223-234. doi: 10.1080/15476286.2023.2216562. RNA Biol. 2023. PMID: 37221841 Free PMC article. Review.
-
Reprogramming of sentinel lymph node microenvironment during tumor metastasis.J Biomed Sci. 2022 Oct 20;29(1):84. doi: 10.1186/s12929-022-00868-1. J Biomed Sci. 2022. PMID: 36266717 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


