Comparative effectiveness of post-discharge interventions for hospitalized smokers: study protocol for a randomized controlled trial
- PMID: 22852832
- PMCID: PMC3487923
- DOI: 10.1186/1745-6215-13-124
Comparative effectiveness of post-discharge interventions for hospitalized smokers: study protocol for a randomized controlled trial
Abstract
Background: A hospital admission offers smokers an opportunity to quit. Smoking cessation counseling provided in the hospital is effective, but only if it continues for more than one month after discharge. Providing smoking cessation medication at discharge may add benefit to counseling. A major barrier to translating this research into clinical practice is sustaining treatment during the transition to outpatient care. An evidence-based, practical, cost-effective model that facilitates the continuation of tobacco treatment after discharge is needed. This paper describes the design of a comparative effectiveness trial testing a hospital-initiated intervention against standard care.
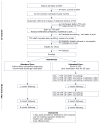
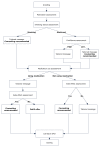
Methods/design: A two-arm randomized controlled trial compares the effectiveness of standard post-discharge care with a multi-component smoking cessation intervention provided for three months after discharge. Current smokers admitted to Massachusetts General Hospital who receive bedside smoking cessation counseling, intend to quit after discharge and are willing to consider smoking cessation medication are eligible. Study participants are recruited following the hospital counseling visit and randomly assigned to receive Standard Care or Extended Care after hospital discharge. Standard Care includes a recommendation for a smoking cessation medication and information about community resources. Extended Care includes up to three months of free FDA-approved smoking cessation medication and five proactive computerized telephone calls that use interactive voice response technology to provide tailored motivational messages, offer additional live telephone counseling calls from a smoking cessation counselor, and facilitate medication refills. Outcomes are assessed at one, three, and six months after hospital discharge. The primary outcomes are self-reported and validated seven-day point prevalence tobacco abstinence at six months. Other outcomes include short-term and sustained smoking cessation, post-discharge utilization of smoking cessation treatment, hospital readmissions and emergency room visits, and program cost per quit.
Discussion: This study tests a disseminable smoking intervention model for hospitalized smokers. If effective and widely adopted, it could help to reduce population smoking rates and thereby reduce tobacco-related mortality, morbidity, and health care costs.
Trial registration: United States Clinical Trials Registry NCT01177176.
Figures
Similar articles
-
Comparative Effectiveness of Post-Discharge Strategies for Hospitalized Smokers: study protocol for the Helping HAND 2 randomized controlled trial.BMC Public Health. 2015 Feb 7;15:109. doi: 10.1186/s12889-015-1484-0. BMC Public Health. 2015. PMID: 25879193 Free PMC article. Clinical Trial.
-
Nicotine patches and quitline counseling to help hospitalized smokers stay quit: study protocol for a randomized controlled trial.Trials. 2012 Aug 1;13:128. doi: 10.1186/1745-6215-13-128. Trials. 2012. PMID: 22853197 Free PMC article. Clinical Trial.
-
Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial.JAMA. 2014 Aug 20;312(7):719-28. doi: 10.1001/jama.2014.9237. JAMA. 2014. PMID: 25138333 Free PMC article. Clinical Trial.
-
Effectiveness and cost-effectiveness of computer and other electronic aids for smoking cessation: a systematic review and network meta-analysis.Health Technol Assess. 2012;16(38):1-205, iii-v. doi: 10.3310/hta16380. Health Technol Assess. 2012. PMID: 23046909 Review.
-
Interventions for Tobacco Cessation in Adults, Including Pregnant Women: An Evidence Update for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 Jan. Report No.: 20-05264-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 Jan. Report No.: 20-05264-EF-1. PMID: 33523610 Free Books & Documents. Review.
Cited by
-
Interventions for smoking cessation in hospitalised patients.Cochrane Database Syst Rev. 2024 May 21;5(5):CD001837. doi: 10.1002/14651858.CD001837.pub4. Cochrane Database Syst Rev. 2024. PMID: 38770804 Review.
-
Smartphone-based financial incentives to promote smoking cessation during pregnancy: A pilot study.Prev Med. 2020 Nov;140:106201. doi: 10.1016/j.ypmed.2020.106201. Epub 2020 Jul 9. Prev Med. 2020. PMID: 32652133 Free PMC article. Clinical Trial.
-
Comparative effectiveness of post-discharge strategies for hospitalized smokers: Study protocol for the Helping HAND 4 randomized controlled trial.Trials. 2020 Apr 16;21(1):336. doi: 10.1186/s13063-020-04257-7. Trials. 2020. PMID: 32299470 Free PMC article.
-
Real-time video counselling for smoking cessation.Cochrane Database Syst Rev. 2019 Oct 29;2019(10):CD012659. doi: 10.1002/14651858.CD012659.pub2. Cochrane Database Syst Rev. 2019. PMID: 31684699 Free PMC article.
-
Adaptation of a sustained care cessation intervention for smokers hospitalized for psychiatric disorders: Study protocol for a randomized controlled trial.Contemp Clin Trials. 2019 Aug;83:18-26. doi: 10.1016/j.cct.2019.06.001. Epub 2019 Jun 15. Contemp Clin Trials. 2019. PMID: 31212100 Free PMC article.
References
-
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. Lung Health Study Research Group: the effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–239. - PubMed
-
- Fiore MC, Jaén CR, Baker TB, Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. MD: US: Department of Health and Human Services. Public Health Service, Rockville ; 2008.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical