Medical methods for mid-trimester termination of pregnancy
- PMID: 21249669
- PMCID: PMC8557267
- DOI: 10.1002/14651858.CD005216.pub2
Medical methods for mid-trimester termination of pregnancy
Abstract
Background: With the improvement of ultrasound technology, the likelihood of detection of major fetal structural anomalies in mid-pregnancy has increased considerably. Upon the detection of serious anomalies, women typically are offered the option of pregnancy termination. Additionally, there are still many reasons other than fetal anomalies why women seek abortion in the mid-trimester.
Objectives: To compare different methods of second trimester medical termination of pregnancy for their efficacy and side-effects.
Search strategy: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE, Popline and reference lists of retrieved papers and other sources.
Selection criteria: All randomised controlled trials (RCTs) examining medical regimens for termination of pregnancy of a singleton living fetus between 12-28 weeks' gestation were analysed. The outcome measures were the induction to abortion interval, abortion rate within 24 hours, need for surgical evacuation, blood loss, uterine rupture, pain, and side-effects.Trials including >20% fetal death, multiple pregnancies, previous uterine scars and regimens which involved cervical preparation were excluded.
Data collection and analysis: Two authors selected the trials and three authors extracted data.
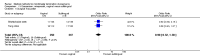
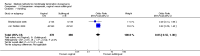
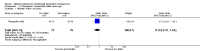
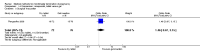
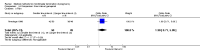
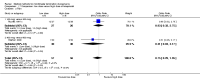
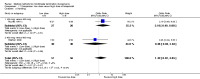
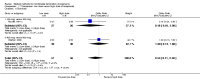
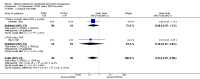
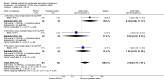
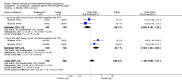
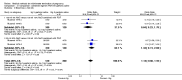
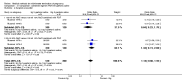
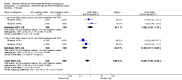
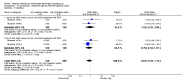
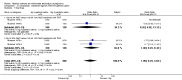
Main results: Fourty RCTs were included, addressing various agents for pregnancy termination and methods of administration. When used alone, misoprostol was an effective inductive agent, though it appeared to be more effective in combination with mifepristone. However, the evidence from RCTs is limited.Misoprostol was preferably administered vaginally, although among multiparous women sublingual administration appeared equally effective. A range of doses of vaginally administered misoprostol has been used. No randomised trials comparing doses of misoprostol were identified; however low doses of misoprostol appear to be associated with fewer side-effects while moderate doses appear to be more efficient in completing abortion. Four RCTs showed that the induction to abortion interval with 3-hourly vaginal administration of prostaglandins is shorter than 6-hourly administration without an increase in side-effects.Many studies reported the need for surgical evacuation. Indications for surgical evacuation include retained products of the placenta and heavy vaginal bleeding. Fewer women required surgical evacuation when misoprostol was administrated vaginally compared with women receiving intra-amniotical PGF(2a) . Mild, self-limiting diarrhoea was more common among women who received misoprostol compared to other agents.
Authors' conclusions: Medical abortion in the second trimester using the combination of mifepristone and misoprostol appeared to have the highest efficacy and shortest abortion time interval. Where mifepristone is not available, misoprostol alone is a reasonable alternative. The optimal route for administering misoprostol is vaginally, preferably using tablets at 3-hourly intervals. Apart from pain, the side-effects of vaginal misoprostol are usually mild and self limiting. Conclusions from this review are limited by the gestational age ranges and variable medical regimens, including dosing, administrative routes and intervals of medication, of the included trials.
Conflict of interest statement
None declared
Figures














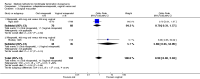
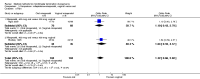

















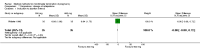













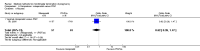





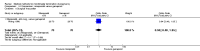
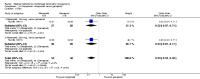

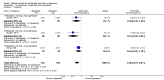
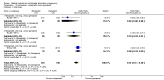
































































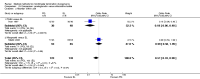

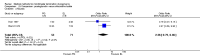


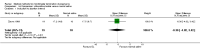




Update of
- doi: 10.1002/14651858.CD005216
Similar articles
-
Medical methods for first trimester abortion.Cochrane Database Syst Rev. 2004;(2):CD002855. doi: 10.1002/14651858.CD002855.pub3. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2011 Nov 09;(11):CD002855. doi: 10.1002/14651858.CD002855.pub4. PMID: 15106180 Updated. Review.
-
Medical methods for first trimester abortion.Cochrane Database Syst Rev. 2011 Nov 9;2011(11):CD002855. doi: 10.1002/14651858.CD002855.pub4. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2022 May 24;5:CD002855. doi: 10.1002/14651858.CD002855.pub5. PMID: 22071804 Free PMC article. Updated. Review.
-
Medical methods for first trimester abortion.Cochrane Database Syst Rev. 2004;(1):CD002855. doi: 10.1002/14651858.CD002855.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2004;(2):CD002855. doi: 10.1002/14651858.CD002855.pub3. PMID: 14973995 Updated. Review.
-
Medical methods for first trimester abortion.Cochrane Database Syst Rev. 2022 May 24;5(5):CD002855. doi: 10.1002/14651858.CD002855.pub5. Cochrane Database Syst Rev. 2022. PMID: 35608608 Free PMC article. Review.
-
Self-administered versus provider-administered medical abortion.Cochrane Database Syst Rev. 2020 Mar 9;3(3):CD013181. doi: 10.1002/14651858.CD013181.pub2. Cochrane Database Syst Rev. 2020. PMID: 32150279 Free PMC article.
Cited by
-
The efficacy of mifepristone-misoprostol regimen versus misoprostol-only for medication abortion at 22 + 0/7 to 30 + 0/7 weeks' gestation.Arch Gynecol Obstet. 2024 Sep 18. doi: 10.1007/s00404-024-07737-2. Online ahead of print. Arch Gynecol Obstet. 2024. PMID: 39292225
-
Dilation and evacuation versus medication abortion at 15-24 weeks of gestation in low-middle income country: A retrospective cohort study.Contracept X. 2024 Aug 8;6:100110. doi: 10.1016/j.conx.2024.100110. eCollection 2024. Contracept X. 2024. PMID: 39281371 Free PMC article.
-
Have we overlooked the role of mifepristone for the medical management of tubal ectopic pregnancy?Hum Reprod. 2023 Aug 1;38(8):1445-1448. doi: 10.1093/humrep/dead116. Hum Reprod. 2023. PMID: 37295950 Free PMC article.
-
Factors Influencing the Duration of Termination of Pregnancy for Fetal Anomaly with Mifepristone in Combination with Misoprostol.J Clin Med. 2023 Jan 21;12(3):869. doi: 10.3390/jcm12030869. J Clin Med. 2023. PMID: 36769518 Free PMC article.
-
Medical abortion at 13 or more weeks gestation provided through telemedicine: A retrospective review of services.Contracept X. 2021 Jan 25;3:100057. doi: 10.1016/j.conx.2021.100057. eCollection 2021. Contracept X. 2021. PMID: 33615210 Free PMC article.
References
References to studies included in this review
Akoury 2004 {published data only}
-
- Akoury HA, Hannah ME, Chitayat D, Thomas M, Winsor E, Ferris LE, et al. Randomized controlled trial of misoprostol for second‐trimester pregnancy termination associated with fetal malformation. American Journal of Obstetrics and Gynecology 2004;190(3):755‐62. - PubMed
Armatage 1996 {published data only}
-
- Armatage RJ, Luckas MJ. A randomized trial of 2 regimens for the administration of vaginal prostaglandins (gemeprost) for the induction of midtrimester abortion. The Australian & New Zealand Journal of Obstetrics & Gynaecology 1996;36(3):296‐9. - PubMed
Bartley 2002 {published data only}
-
- Bartley J, Baird DT. A randomised study of misoprostol and gemeprost in combination with mifepristone for induction of abortion in the second trimester of pregnancy. BJOG 2002;109(11):1290‐4. - PubMed
Bebbington 2002 {published data only}
-
- Bebbington MW, Kent N, Lim K, Gagnon A, Delisle MF, Tessier F, et al. A randomized controlled trial comparing two protocols for the use of misoprostol in midtrimester pregnancy termination. American Journal of Obstetrics and Gynecology 2002;187(4):853‐7. - PubMed
Behrashi 2008 {published data only}
-
- Behrashi M, Mahdian M. Vaginal versus oral misoprostol for second trimester pregnancy termination: a randomised trial. Pakistan Journal of Biological Sciences 2008;11(21):2505‐8. - PubMed
Bhattacharjee 2008 {published data only}
-
- Bhattacharjee N, Saha SP, Ghosroy SC, Bhowmik S, Barui G. A randomised comparative study on sublingual versus vaginal administration of misoprostol for termination of pregnancy between 13 to 20 weeks. The Australian & New Zealand Journal of Obstetrics & Gynaecology 2008;48:165‐71. - PubMed
Borgida 1995 {published data only}
-
- Borgida AF, Rodis JF, Hanlon W, Craffey A, Ciarleglio L, Campbell WA. Second‐trimester abortion by intramuscular 15‐methyl‐prostaglandin F2 alpha or intravaginal prostaglandin E2 suppositories: a randomized trial. Obstetrics and Gynecology 1995;85(5):697‐700. - PubMed
Chai 2009 {published data only}
-
- Chai J, Tang OS, Hong QQ, Chen QF, Cheng LN, Ng E, Ho PC. A randomized trial to compare two dosing intervals of misoprostol following mifepristone administration in second trimester medical abortion. Human Reproduction 2009;24(2):320‐4. - PubMed
el‐Refaey 1993 {published data only}
-
- El‐Refaey H, Hinshaw K, Templeton A. The abortifacient effect of misoprostol in the second trimester. A randomized comparison with gemeprost in patients pre‐treated with mifepristone (RU486). Human Reproduction 1993;8(10):1744‐6. - PubMed
El‐refaey 1995 {published data only}
-
- El‐Refaey H, Templeton A. Induction of abortion in the second trimester by a combination of misoprostol and mifepristone: a randomized comparison between two misoprostol regimens. Human Reproduction 1995;10(2):475‐8. - PubMed
Faktor 1988 {published data only}
-
- Faktor JH, Frenkel Y, Mashiach S, Serr DM. Intra‐amniotic injection of oxytetracycline hydrochloride for termination of mid trimester abortion. Gynecologic and Obstetric Investvestigation 1988;26:177‐80. - PubMed
Hamoda 2005 {published data only}
-
- Hamoda H, Ashok PW, Flett GMM, Templeton A. A randomized trial of mifepristone in combination with misoprostol administered sublingually or vaginally for medical abortion at 13‐20 weeks gestation. Human Reproduction 2005;20(8):2348‐54. - PubMed
Herabutya 2005 {published data only}
-
- Herabutya Y, Chanrachakul B, Punyavachira P. A randomised controlled trial of 6 and 12 hourly administration of vaginal misoprostol for second trimester pregnancy termination. BJOG 2005;112(9):1297‐301. - PubMed
Ho 1996 {published data only}
-
- Ho PC, Chan JF, Lau W. Misoprostol is as effective as gemeprost in termination of second trimester pregnancy when combined with mifepristone: a randomised comparative trial. Contraception 1996;53(5):281‐3. - PubMed
Ho 1997 {published data only}
-
- Ho PC, Ngai SW, Liu KL, Wong GC, Lee SW. Vaginal misoprostol compared with oral misoprostol in termination of second‐trimester pregnancy. Obstetrics and Gynecology 1997;90(5):735‐8. - PubMed
Inan 1997 {published data only}
-
- Inan I, Kelekci S, Yazar D. Comparison of ethacridine lactate and prostaglandin E2 in second trimester medical abortion. Acta Obstetricia et Gynecologica Scandinavica 1996;76:680‐3. - PubMed
Kapp 2007 {published data only}
-
- Kapp N, Borgatta L, Stubblefield P, Vragovic O, Moreno N. Mifepristone in second‐trimester medical abortion: A randomized controlled trial. Obstetrics and Gynecology 2007;110(6):1304‐10. - PubMed
Kelekci 2006 {published data only}
-
- Kelekci S, Erdemoglu E, Inan I. Randomized study on the effect of adding oxytocin to ethacridine lactate or misoprostol for second‐trimester termination of pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2006;85:825‐9. - PubMed
Makhlouf 2003 {published data only}
-
- Makhlouf AM, Al‐Hussaini TK, Habib DM, Makarem MH. Second‐trimester pregnancy termination: comparison of three different methods. Journal of Obstetrics and Gynaecology 2003;23(4):407‐11. - PubMed
Mehta 1975 a {published data only}
-
- Mehta A, Ghatge V, Dave S, Chaina M, Shah P. Termination of second trimester pregnancies: a blind study using hypertonic saline and prostaglandins F2a ‐ a preliminary report. Journal of Obstetrics and Gynaecology India 1975;25(2):165‐75. - PubMed
Mehta 1975 b {published data only}
-
- Mehta A, Ghatge V, Dave S, CHaina M, Shah P. Termination of second trimester pregnancies: a blind study using hypertonic saline and prostaglandins F2a ‐ a preliminary report. Journal of Obstetrics and Gynaecology India 1975;25(2):165‐75. - PubMed
Muzsnai 1979 a {published data only}
-
- Muzsnai D, Kerenyi T. Use of prostaglandin, hypertonic saline and oxytocin for second‐trimester abortion. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1979;9/6:385‐9. - PubMed
Muzsnai 1979 b {published data only}
-
- Muzsnai D, Kerenyi T. Use of prostaglandin, hypertonic saline and oxytocin for second‐trimester abortion. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1979;9/6:385‐9. - PubMed
Muzsnai 1979 c {published data only}
-
- Muzsnai D, Kerenyi T. Use of prostaglandin, hypertonic saline and oxytocin for second‐trimester abortion. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1979;9/6:385‐9. - PubMed
Muzsnai 1979 d {published data only}
-
- Muzsnai D, Kerenyi T. Use of prostaglandin, hypertonic saline and oxytocin for second‐trimester abortion. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1979;9/6:385‐9. - PubMed
Ngai 2000 {published data only}
-
- Ngai SW, Tang OS, Ho PC. Randomized comparison of vaginal (200 µg every 3 hrs) and oral (400 µg every 3 hrs) misoprostol when combined with mifepristone in termination of second trimester pregnancy. Human Reproduction 2000;15(10):2205‐8. - PubMed
Nielsen 1975 {published data only}
-
- Nielsen KR, Gregersen E, Larsen JF, Olsen CE. Prostaglandin F2alpha and oxytocin compared with hypertonic saline and oxytocin for the induction of second trimester abortion.. Acta Obstetricia et Gynecologica Scandinavica. Supplement 1975;37:57‐60. - PubMed
Nuutila 1997 a {published data only}
-
- Nuutila M, Toivonen J, Ylikorkala O, Halmesmäki E. A comparison between two doses of intravaginal misoprostol and gemeprost for induction of second‐trimester abortion. Obstetrics and Gynecology 1997;90(6):896‐900. - PubMed
Nuutila 1997 b {published data only}
-
- Nuutila M, Toivonen J, Ylikorkala O, Halmesmäki E. A comparison between two doses of intravaginal misoprostol and gemeprost for induction of second‐trimester abortion. Obstetrics and Gynecology 1997;90(6):896‐900. - PubMed
Nuutila 1997 c {published data only}
-
- Nuutila M, Toivonen J, Ylikorkala O, Halmesmäki E. A comparison between two doses of intravaginal misoprostol and gemeprost for induction of second‐trimester abortion. Obstetrics and Gynecology 1997;90(6):896‐900. - PubMed
Olund 1978 {published data only}
-
- Olund A, Larsson B. Comparison of extra‐amniotic instillation of rivanol and PGF2a either separately or in combination followed by oxytocin for second trimester abortion. Acta Obstetricia et Gynecologica Scandinavica 1978;57:333‐6. - PubMed
Ozerkan 2009 {published data only}
-
- Ozerkan K, Ocakoglu G, Rehimli S, Uncu G, Develioglu O. A comparison of low‐dose and high‐dose protocols of vaginal misoprostol for second trimester termination of pregnancy. Clinical and Experimental Obstetrics & Gynecology 2009;36(4):245‐7. - PubMed
Pongsatha 2008 {published data only}
-
- Pongsatha S, Tongsong T. Randomized comparison of dry tablet insertion versus gel form of vaginal misoprostol for second trimester pregnancy termination. The Journal of Obstetrics and Gynaecology Research 2008;34(2):199‐203. - PubMed
Sorensen 1984 {published data only}
-
- Sorensen SS, Wolf P. Randomized trial of intracervical prostaglandin E2 gel and intra‐amniotic prostaglandin F2alfa for induction of second trimester abortion. Contraception 1984;29(2):171‐9. - PubMed
Steyn 1993 {published data only}
-
- Steyn DW, Pienaar MP. Mid‐trimester termination of pregnancy‐‐a randomised controlled trial of two prostaglandin regimens. South African Medical Journal 1993;83(10):737‐8. - PubMed
Su 2005 {published data only}
-
- Su LL, Biswas A, Choolani M, Kalaichelvan V, Singh K. A prospective, randomized comparison of vaginal misoprostol versus intra‐amniotic prostaglandins for midtrimester termination of pregnancy. American Journal of Obstetrics and Gynecology 2005;193(4):1410‐4. - PubMed
Tang 2004 {published data only}
-
- Tang OS, Chan CC, Kan AS, Ho PC. A prospective randomized comparison of sublingual and vaginal misoprostol in second trimester termination of pregnancy. BJOG 2004;111:1001‐5. - PubMed
Tang 2005 {published data only}
-
- Tang OS, Chan CC, Kan AS, Ho PC. A prospective randomized comparison of sublingual and oral misoprostol when combined with mifepristone for medical abortion at 12‐20 weeks gestation. Human Reproduction 2005;20(11):3062‐6. - PubMed
Thong 1996 {published data only}
-
- Thong KJ, Lynch P, Baird DT. A randomised study of two doses or gemeprost in combination with mifepristone for induction of abortion in the second trimester of pregnancy. Contraception 1996;54(2):97‐100. - PubMed
von Hertzen 2009 {published data only}
-
- Hertzen H, Piaggio G, Wojdyla D, Nguyen TM, Marions L, Okoev G, et al. WHO Research Group on Post‐ovulatory Methods of Fertility Regulation. Comparison of vaginal and sublingual misoprostol for second trimester abortion: randomised controlled equivalence trial. Human Reproduction 2009;24(1):106‐12. - PubMed
Waldron 1990 {published data only}
-
- Waldron KW, Renou PM, Lolatgis N, Morris ND, Mamers PM, Oldham J, Healy DL. Second‐trimester termination with 16,16 dimethyl‐PGE1‐methyl ester (gemeprost) compared with a regimen that included intra‐amniotic PGF2a and hypertonic saline. Reproduction, Fertility, and Development 1990;2:495‐8. - PubMed
Webster 1996 {published data only}
-
- Webster D, Penney GC, Templeton A. A comparison of 600 and 200 mg mifepristone prior to second trimester abortion with the prostaglandin misoprostol. British Journal of Obstetrics and Gynaecology 1996;103(7):706‐9. - PubMed
WHO 1976 {published data only}
Wong 1998 {published data only}
-
- Wong KS, Ngai CS, Wong AY, Tang LC, Ho PC. Vaginal misoprostol compared with vaginal gemeprost in termination of second trimester pregnancy. A randomized trial. Contraception 1998;58(4):207‐10. - PubMed
Wong 2000 {published data only}
-
- Wong KS, Ngai CS, Yeo EL, Tang LC, Ho PC. A comparison of two regimens of intravaginal misoprostol for termination of second trimester pregnancy: a randomized comparative trial. Human Reproduction 2000;15(3):709‐12. - PubMed
Zauva 1989 {published data only}
-
- Zauva BL, Gupta I, Dhall GI. Mid‐trimester abortion by extra‐amniotic emcredil versus normal saline. The Australian & New Zealand Journal of Obstetrics & Gynaecology 1989;29(3(2)):299‐302. - PubMed
References to studies excluded from this review
Allahbadia 1992 {published data only}
-
- Allahbadia G. Comparative study of midtrimester termination of pregnancy using hypertonic saline, ethacridine lactate, prostaglandin analogue and iodine‐saline. Journal of the Indian Medical Association 1992;90(9):237‐9. - PubMed
Ballard 1981 {published data only}
-
- Ballard CA. The vaginal administration of 9‐deoxo‐16,16‐dimethyl‐9‐methylene PGE2 for second trimester abortion. Contraception 1981;24(2):151‐7. - PubMed
Ben‐Meir 2009 {published data only}
-
- Ben‐Meir A, Erez Y, Feigenberg T, Hamani Y, Laufer N, Rojansky N. Mifepristone followed by high‐dose oxytocin drip for second‐trimester abortion. The Journal of Reproductive Medicine 2009;54(8):511‐6. - PubMed
Caliskan 2005 {published data only}
-
- Caliskan E, Dilbaz S, Doger E, Ozeren S, Dilbaz B. Randomized comparison of 3 misoprostol protocols for abortion induction at 13‐20 weeks of gestation. The Journal of Reproductive Medicine 2005;50(3):173‐80. - PubMed
Caliskan 2009 {published data only}
-
- Caliskan E, Doger E, Cakiroglu Y, Corakci A, Yucesoy I. Sublingual misoprostol 100 microgram versus 200 microgram for second trimester abortion: a randomised trial. The European Journal of Contraception & Reproductive Health Care 2009;14(1):55‐60. - PubMed
Carbonell 2008 {published data only}
-
- Carbonell JL, Torres MA, Reyes R, Ortega L, García‐Gallego F, Sánchez C. Second‐trimester pregnancy termination with 600‐μg vs. 400‐μg vaginal misoprostol and systematic curettage post expulsion: a randomized trial. Contraception 2007;77(1):50‐5. - PubMed
Dickinson 1998 {published data only}
-
- Dickinson JE, Godfrey M, Evans SF. Efficacy of intravaginal misoprostol in second‐trimester pregnancy termination: a randomized controlled trial. The Journal of Maternal‐Fetal Medicine 1998;7(3):115‐9. - PubMed
Dickinson 2002 {published data only}
-
- Dickinson JE, Evans SF. The optimization of intravaginal misoprostol dosing schedules in second trimester pregnancy termination. American Journal of Obstetrics and Gynecology 2002;186(3):470‐4. - PubMed
Dickinson 2003 {published data only}
-
- Dickinson JE, Evans SF. A comparison of oral misoprostol with vaginal misoprostol administration in second‐trimester pregnancy termination for fetal abnormality. Obstetrics and Gynecology 2003;101(6):1294‐9. - PubMed
Feldman 2003 {published data only}
-
- Feldman DM, Borgida AF, Rodis JF, Leo MV, Campbell WA. A randomized comparison of two regimens of misoprostol for second‐trimester pregnancy termination. American Journal of Obstetrics and Gynecology 2003;189(3):710‐3. - PubMed
Frydman 1988 {published data only}
-
- Frydman R, Fernandez H, Pons JC, Ulmann A. Mifepristone (RU486) and therapeutic late pregnancy termination: a double‐blind study of two different doses. Human Reproduction 1988;3(6):803‐6. - PubMed
Ghorab 1998 {published data only}
-
- Ghorab MN, Helw BA. Second‐trimester termination of pregnancy by extra‐amniotic prostaglandin F2alpha or endocervical misoprostol. A comparative study. Acta Obstetricia et Gynecologica Scandinavica 1998;77(4):429‐32. - PubMed
Ghosh 1980 {published data only}
-
- Ghodh AK, Konar JR. The relative value of two concentrations of hypertonic saline for midtrimester abortion. International Journal of Gynaecology and Obstetrics 1980;17:368‐71. - PubMed
Gilbert 2001 {published data only}
-
- Gilbert A, Reid R. A randomised trial of oral versus vaginal administration of misoprostol for the purpose of mid‐trimester termination of pregnancy. The Australian & New Zealand Journal of Obstetrics & Gynaecology 2001;41(4):407‐10. - PubMed
Goswami 1982 {published data only}
-
- Goswami BK, Raha A, Gupta A, Mukherjee K. Midtrimester abortion by ethacridine lactate. Journal of the Indian Medical Association 1982;79(1‐2):7‐9. - PubMed
Guix 2005 {published data only}
-
- Guix C, Palacio M, Figueras F, Bennasar M, Zamora L, Coll O, et al. Efficacy of two regimens of misoprostol for early second‐trimester pregnancy termination. Fetal Diagnosis and Therapy 2005;20(6):544‐8. - PubMed
Herabutya 2001 {published data only}
-
- Herabutya Y, Chanrachakul B, Punyavachira P. Second trimester pregnancy termination: a comparison of 600 and 800 micrograms of intravaginal misoprostol. The Journal of Obstetrics and Gynaecology Research 2001;27(3):125‐8. - PubMed
Hidar 2001 {published data only}
-
- Hidar S, Fekih M, Chaieb, Bibi M, Mellouli R, Khairi H. Oxytocin and misoprostol administered intravaginally for termination of pregnancy at 13 to 29 weeks of amenorrhea. A prospective randomized trial [Apport de l'association d'oxytocine au misoprostol administre en intravaginal au cours des interruptions de grossesses entre 13 et 29 semaines d'amenorrhee]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2001;30(5):439‐43. - PubMed
Hill 1991 {published data only}
-
- Hill NC, Selinger M, Ferguson J, MacKenzie IZ Hill NC, Selinger M, Ferguson J, et al. Mid‐trimester termination of pregnancy with 16,16‐dimethyl‐trans‐delta 2 PGE1 vaginal pessaries: a comparison with intra‐ and extra‐amniotic prostaglandin E2 administration. International Journal of Gynaecology and Obstetrics 1991;35(4):337‐40. - PubMed
Ho 1993 {published data only}
-
- Ho PC, Ma HK. Termination of second trimester pregnancy with sulprostone and mifepristone: a randomized double‐blind placebo‐controlled trial. Contraception 1993;47:123‐9. - PubMed
Jain 1994 {published data only}
-
- Jain JK, Mishell DR Jr. A comparison of intravaginal misoprostol with prostaglandin E2 for termination of second‐trimester pregnancy. The New England Journal of Medicine 1994;331(5):290‐3. - PubMed
Jain 1999 {published data only}
-
- Jain JK, Kuo J, Mishell DR Jr. A comparison of two dosing regimens of intravaginal misoprostol for second‐trimester pregnancy termination. Obstetrics and Gynecology 1999;93(4):571‐5. - PubMed
Jansen 2008 {published data only}
-
- Jansen NE, Pasker‐De Jong PC, Zondervan HA. Mifepristone and misoprostol versus dilapan and sulprostone for second trimester termination of pregnancy. The Journal of Maternal‐Fetal & Neonatal Medicine 2008;21(11):847‐51. - PubMed
Jarnbert 1999 {published data only}
-
- Järnbert A, Klang B, Thi Vinh N, Ngoc Hamb N. Comparative study of cervical laminar tents prior to extra‐amniotic injection of ethacridine lactate (rivanol) and a condom‐nelathon catheter method for second‐trimester pregnancy interruption in Vietnam. Gynecologic and Obstetric Investigation 1999;48:113‐8. - PubMed
Kamali 1998 {published data only}
-
- Kamali P, Hohmann M, Herrero J, Künzel W. Gemeprost, sulprostone and dinoprostone for induced abortion in the 15th‐24th week of pregnancy. Zentralblatt fur Gynakologie 1998;120(6):293‐300. - PubMed
Kapp 2007 (2) {published data only}
-
- Kapp N, Todd CS, Yadgarova KT, Alibayeva G, Nazarova D, Loza O, Babadjanova GS. A randomized comparison of misoprostol to intrauterine instillation of hypertonic saline plus a prostaglandin F2α analogue for second‐trimester induction termination in Uzbekistan. Contraception 2007;76(6):461‐6. - PubMed
Klinte 1983 {published data only}
-
- Klinte I, Hamberger L, Wiqvist N. Second‐trimester abortion by extra‐amniotic instillation of Rivanol combined with intravenous administration of oxytocin or prostaglandin F2 alpha. Acta Obstetricia et Gynecologica Scandinavica 1983;62(4):303‐6. - PubMed
le Roux 2002 {published data only}
-
- Roux PA, Tregoning SK, Zinn PM, Spuy ZM. Inhibition of progesterone secretion with trilostane for midtrimester termination of pregnancy: randomized controlled trials. Human Reproduction 2002;17(6):1483‐9. - PubMed
Manabe 1981 {published data only}
-
- Manabe Y, Manabe A. Abortion during mid‐pregnancy by rivanol‐catheter supplemented with PGF2a drip‐infusion or quinine hydrochloride. Contraception 1981;23(6):621‐8. - PubMed
Munthali 2001 {published data only}
-
- Munthali J, Moodley J. The use of misoprostol for mid‐trimester therapeutic termination of pregnancy. Tropical Doctor 2001;31(3):157‐61. - PubMed
Nigam 2006 {published data only}
-
- Nigam A, Singh VK, Prakash A. Vaginal vs. oral misoprostol for mid‐trimester abortion. International Journal of Gynaecology and Obstetrics 2006;92(3):270‐1. - PubMed
Niromanesh 2005 {published data only}
-
- Niromanesh S, Hashemi‐Fesharaki M, Mosavi‐Jarrahi A. Second trimester abortion using intravaginal misoprostol [brief communication]. International Journal of Gynaecology and Obstetrics 2005;89(3):276‐7. - PubMed
Nor Azlin 2006 {published data only}
-
- Nor Azlin MI, Abdullah HS, Zainul Rashid MR, Jamil MA. Misoprostol (alone) in second trimester terminations of pregnancy: as effective as gemeprost?. Journal of Obstetrics and Gynaecology 2006;26(6):546‐9. - PubMed
Nuthalapaty 2005 {published data only}
Olund 1979 {published data only}
-
- Olund A. The effect of indomethacin on the instillation‐abortion interval in rivanol‐induced mid‐trimester abortion. Acta Obstetricia et Gynecologica Scandinavica 1979;58(1):121‐2. - PubMed
Owen 1996 {published data only}
-
- Owen J, Hauth JC. Concentrated oxytocin plus low‐dose prostaglandin E2 compared with prostaglandin E2 vaginal suppositories for second‐trimester pregnancy termination. Obstetrics and Gynecology 1996;88(1):110‐3. - PubMed
Owen 1999 {published data only}
-
- Owen J, Hauth JC. Vaginal misoprostol vs. concentrated oxytocin plus low‐dose prostaglandin E2 for second trimester pregnancy termination. J Matern Fetal Med 1999;8(2):48‐50. - PubMed
Perry 1999 {published data only}
-
- Perry KG Jr, Rinehart BK, Terrone DA, Martin RW, May WL, Roberts WE. Second‐trimester uterine evacuation: A comparison of intra‐amniotic (15S)‐15‐methyl‐prostaglandin F2alpha and intravaginal misoprostol. American Journal of Obstetrics and Gynecology 1999;181(5):1057‐61. - PubMed
Pulkkinen 1980 {published data only}
-
- Pulkkinen MO, Kajanoja P, Kivikoski A, Saastamoinen J, Selander K, Tuimala R. Abortion with sulprostone, a prostaglandin E2 derivative. International Journal of Gynaecology and Obstetrics 1980;18(1):40‐3. - PubMed
Ragab 1976 {published data only}
-
- Ragab MI, Edelman DA. Midtrimester abortion: a comparison of intra‐amniotic Prostaglandin F2a and hypertonic saline. International Journal of Gynaecology and Obstetrics 1976;14:393‐6. - PubMed
Ramsey 2004 {published data only}
-
- Ramsey PS, Savage K, Lincoln T, Owen J. Vaginal misoprostol versus concentrated oxytocin and vaginal PGE2 for second trimester labor induction. Obstetrics and Gynecology 2004;104(1):138‐45. - PubMed
Shukla 1984 {published data only}
-
- Shukla S, Sapre S, Olyai P. Mid‐trimester pregnancy termination with ethacridine lactate. Journal of the Indian Medical Association 1984;82(12):432‐4. - PubMed
Sørensen 1986 {published data only}
-
- Stampe Sørensen S, Heisterberg L, Wolf P. Midtrimester abortion by intracervical prostaglandin E2. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1986;21(3):165‐71. - PubMed
Thong 1993 {published data only}
-
- Thong KJ, Baird DT. Induction of second trimester abortion with mifepristone and gemeprost. British Journal of Obstetrics and Gynaecology 1993;100(8):758‐61. - PubMed
Thong KJ, Baird 1992 {published data only}
-
- Thong KJ, Baird DT. A study of gemeprost alone, dilapan of mifepristone in combination with gemeprost for the termination of second trimester pregnancy. Contraception 1992;46:11‐7. - PubMed
Wong 1996 {published data only}
-
- Wong KS, Ngai CS, Chan KS, Tang LC, Ho PC. Wong KS, Ngai CS, et al. Termination of second trimester pregnancy with gemeprost and misoprostol: a randomized double‐blind placebo‐controlled trial. Contraception 1996;54(1):23‐5. - PubMed
Yapar 1996 {published data only}
-
- Yapar EG, Senoz S, Orkiittir M, Batioglu S, Gokmen O. Second trimester pregnancy termination including fetal death:comparison of five different methods. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1996;69:97‐102. - PubMed
Yilmaz 2007 {published data only}
-
- Yilmaz B, Kelekci S, Ertas IE, Ozel M, Sut N, Mollamahmutoglu L, Danisman N. Randomized comparison of second trimester pregnancy termination utilizing saline moistened or dry misoprostol. Archives of Gynecology and Obstetrics 2007;276(5):511‐6. - PubMed
Additional references
Akgun 2007
-
- Akgun H, Basbug M, Ozgun MT, Canoz O, Tokat F, Murat N, Ozturk F. Correlation between prenatal ultrasound and fetal autopsy findings in fetal anomalies terminated in the second trimester. Prenatal Diagnosis 2007;27(5):457‐62. - PubMed
Asch 1999
Ballantyne 2009
-
- Ballantyne A, Newson A, Luna F, Ashcroft R. Prenatal diagnosis and abortion for congenital abnormalities: is it ethical to provide one without the other?. The American Journal of Bioethics 2009;9(8):48‐56. - PubMed
Belanger 1981
-
- Bélanger A, Philibert D, Teutsch G. Regio and stereospecific synthesis of 11beta‐substituted 19‐norsteroids. Steroids 1981;37(4):361‐82. - PubMed
Berghella 2009
-
- Berghella V, Airoldi J, O'Neill AM, Einhorn K, Hoffman M. Misoprostol for second trimester pregnancy termination in women with prior caesarean: a systematic review. BJOG 2009;116(9):1151‐7. - PubMed
Boyd 2008
-
- Boyd PA, Devigan C, Khoshnood B, Loane M, Garne E, Dolk H, EUROCAT WorkingGroup. Survey of prenatal screening policies in Europe for structural malformations and chromosome anomalies, and their impact on detection and termination rates for neural tube defects and Down's syndrome. BJOG 2008;115(6):689‐96. - PMC - PubMed
Bygdeman 1985
-
- Bygdeman M, Swahn ML. Progesterone receptor blockage. Effect on uterinecontractility and early pregnancy. Contraception 1985;32(1):45‐51. - PubMed
Bygdeman and Gemzell‐Danielsson 2008
-
- Bygdeman M, Gemzell‐Danielsson K. An historical overview of second trimester abortion methods. Reproductive Health Matters 2008;16 Suppl(31):196‐204. - PubMed
Christin‐Maitre 2000
-
- Christin‐Maitre S, Bouchard P, Spitz IM. Medical termination of pregnancy. The New England Journal of Medicine 2000;342(13):946‐56. - PubMed
Drey 2006
-
- Drey EA, Foster DG, Jackson RA, Lee SJ, Cardenas LH, Darney PD. Risk factors associated with presenting for abortion in the second trimester. Obstetrics and Gynecology 2006;107(1):128‐35. - PubMed
Friedman 2001
-
- Friedman MA. Manufacturer's warning regarding unapproved uses of misoprostol. The New England Journal of Medicine 2001;344(1):61. - PubMed
Goldberg 2001
-
- Goldberg AB, Greenberg MB, Darney PD. Misoprostol and pregnancy. The New England Journal of Medicine 2001;344(1):38‐47. - PubMed
Grimes 1998
-
- Grimes DA. The continuing need for late abortions. JAMA 1998;280(8):747‐50. - PubMed
Higgins 2003
Ho 2007
-
- The continuing need for late abortions. Misoprostol for the termination of pregnancy with a live fetus at 13 to 26 weeks. International Journal of Gynaecology and Obstetrics 2007;99 Suppl 2:178‐81. - PubMed
Hou 2010
-
- Hou S, Chen Q, Zhang L, Fang A, Cheng L. Mifepristone combined with misoprostol versus intra‐amniotic injection of ethacridine lactate for the termination of second trimester pregnancy: a prospective, open‐label, randomized clinical trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2010;151(2):149‐53. - PubMed
Ingham 2008
-
- Ingham R, Lee E, Clements SJ, Stone N. Reasons for second trimester abortions in England and Wales. Reproductive Health Matters 2008;1631 Suppl:18‐29. - PubMed
Isaksen 1998
-
- Isaksen CV, Eik‐Nes SH, Blaas HG, Torp SH. Comparison of prenatal ultrasound and postmortem findings in fetuses and infants with central nervous system anomalies. Ultrasound in Obstetrics & Gynecology 1998;11(4):246‐53. - PubMed
Isaksen 1999
-
- Isaksen CV, Eik‐Nes SH, Blaas HG, Tegnander E, Torp SH. Comparison of prenatal ultrasound and postmortem findings in fetuses and infants with congenital heart defects. Ultrasound in Obstetrics & Gynecology 1999;13(2):117‐26. - PubMed
Kaasen 2006
-
- Kaasen A, Tuveng J, Heiberg A, Scott H, Haugen G. Correlation between prenatal ultrasound and autopsy findings: A study of second‐trimester abortions. Ultrasound in Obstetrics & Gynecology 2006;28(7):925‐33. - PubMed
Lohr 2008
-
- Lohr PA, Hayes JL, Gemzell‐Danielsson K. Surgical versus medical methods for second trimester induced abortion. Cochrane Database of Systematic Reviews 2008;23(1):CD006714. - PubMed
Norman 1992
-
- Norman JE, Thong KJ, Rodger MW, Baird DT. Medical abortion in women of less than or equal to 56 days amenorrhoea: a comparison between gemeprost (a PGE1 analogue) alone and mifepristone and gemeprost. British Journal of Obstetrics and Gynaecology 1992;99(7):601‐6. - PubMed
Swahn 1988
-
- Swahn ML, Johannisson E, Daniore V, Torre B, Bygdeman M. The effect of RU486 administered during the proliferative and secretory phase of the cycle on the bleeding pattern, hormonal parameters and the endometrium. Human Reproduction 1988;3(7):915‐21. - PubMed
Tang 2002
-
- Tang OS, Schweer H, Seyberth HW, Lee SWH, Ho PC. Pharmacokinetics of different routes of administration of misoprostol. Human Reproduction 2002;17:332‐6. - PubMed
Tang 2007
-
- Tang OS, Gemzell‐Danielsson K, Ho PC. Misoprostol: pharmacokinetic profiles,effects on the uterus and side‐effects. Int J Gynaecol Obstet. 2007;99 Suppl 2:S160‐7. - PubMed
Ulmann 1992
-
- Ulmann A, Silvestre L, Chemama L, Rezvani Y, Renault M, Aguillaume CJ, Baulieu EE. Medical termination of early pregnancy with mifepristone (RU 486) followed by a prostaglandin analogue. Study in 16,369 women. Acta Obstetricia et Gynecologica Scandinavica 1992;71(4):278‐83. - PubMed
Wagner 2005
-
- Wagner M. Off‐label use of misoprostol in obstetrics: a cautionary tale. BJOG 2005;112(3):266‐8. - PubMed
Weeks 2005
-
- Weeks AD, Fiala C, Safar P. Misoprostol and the debate over off‐label drug use. BJOG 2005;112(3):269‐72. - PubMed
Zieman 1997
-
- Zieman M, Fong SK, Benowitz NL, Banskter D, Darney PD. Absorption kinetics of misoprostol with oral or vaginal administration. Obstetrics and Gynecology 1997;90(1):88‐92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical


