Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils
- PMID: 19561614
- PMCID: PMC2785232
- DOI: 10.1038/ni.1748
Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils
Abstract
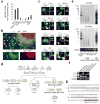
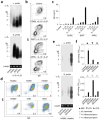
Immunoglobulin D (IgD) is an enigmatic antibody isotype that mature B cells express together with IgM through alternative RNA splicing. Here we report active T cell-dependent and T cell-independent IgM-to-IgD class switching in B cells of the human upper respiratory mucosa. This process required activation-induced cytidine deaminase (AID) and generated local and circulating IgD-producing plasmablasts reactive to respiratory bacteria. Circulating IgD bound to basophils through a calcium-mobilizing receptor that induced antimicrobial, opsonizing, inflammatory and B cell-stimulating factors, including cathelicidin, interleukin 1 (IL-1), IL-4 and B cell-activating factor (BAFF), after IgD crosslinking. By showing dysregulation of IgD class-switched B cells and 'IgD-armed' basophils in autoinflammatory syndromes with periodic fever, our data indicate that IgD orchestrates an ancestral surveillance system at the interface between immunity and inflammation.
Figures

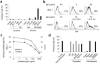


Similar articles
-
The function and regulation of immunoglobulin D.Curr Opin Immunol. 2011 Jun;23(3):345-52. doi: 10.1016/j.coi.2011.01.006. Epub 2011 Feb 24. Curr Opin Immunol. 2011. PMID: 21353515 Free PMC article. Review.
-
New insights into the enigma of immunoglobulin D.Immunol Rev. 2010 Sep;237(1):160-79. doi: 10.1111/j.1600-065X.2010.00929.x. Immunol Rev. 2010. PMID: 20727035 Free PMC article. Review.
-
Secreted IgD Amplifies Humoral T Helper 2 Cell Responses by Binding Basophils via Galectin-9 and CD44.Immunity. 2018 Oct 16;49(4):709-724.e8. doi: 10.1016/j.immuni.2018.08.013. Epub 2018 Oct 2. Immunity. 2018. PMID: 30291028 Free PMC article.
-
Progression from IgD+ IgM+ to isotype-switched B cells is site specific during coronavirus-induced encephalomyelitis.J Virol. 2014 Aug;88(16):8853-67. doi: 10.1128/JVI.00861-14. Epub 2014 May 28. J Virol. 2014. PMID: 24872583 Free PMC article.
-
Responsiveness of B cells is regulated by the hinge region of IgD.Nat Immunol. 2015 May;16(5):534-43. doi: 10.1038/ni.3141. Epub 2015 Apr 6. Nat Immunol. 2015. PMID: 25848865
Cited by
-
Immunoglobulin class-switch recombination: Mechanism, regulation, and related diseases.MedComm (2020). 2024 Aug 13;5(8):e662. doi: 10.1002/mco2.662. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39144468 Free PMC article. Review.
-
The ontogenesis and heterogeneity of basophils.Discov Immunol. 2024 Feb 2;3(1):kyae003. doi: 10.1093/discim/kyae003. eCollection 2024. Discov Immunol. 2024. PMID: 38567293 Free PMC article. Review.
-
IgD+IgM- B Cells in Common Variable Immunodeficiency.Pathogens. 2024 Feb 1;13(2):136. doi: 10.3390/pathogens13020136. Pathogens. 2024. PMID: 38392874 Free PMC article.
-
The multilevel extensive diversity across the cynomolgus macaque captured by ultra-deep adaptive immune receptor repertoire sequencing.Sci Adv. 2024 Jan 26;10(4):eadj5640. doi: 10.1126/sciadv.adj5640. Epub 2024 Jan 24. Sci Adv. 2024. PMID: 38266093 Free PMC article.
-
A Germinal Center Checkpoint of AIRE in B Cells Limits Antibody Diversification.bioRxiv [Preprint]. 2024 Jan 12:2024.01.10.574926. doi: 10.1101/2024.01.10.574926. bioRxiv. 2024. PMID: 38260362 Free PMC article. Preprint.
References
-
- Butler JE, Sun J, Navarro P. The swine Ig heavy chain locus has a single JH and no identifiable IgD. Int Immunol. 1996;8:1897–1904. - PubMed
-
- Preud’homme JL, et al. Structural and functional properties of membrane and secreted IgD. Mol Immunol. 2000;37:871–887. - PubMed
-
- Bengten E, et al. The IgH locus of the channel catfish, Ictalurus punctatus, contains multiple constant region gene sequences: different genes encode heavy chains of membrane and secreted IgD. J Immunol. 2002;169:2488–2497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI057653-03/AI/NIAID NIH HHS/United States
- T32 AI07621/AI/NIAID NIH HHS/United States
- P01 AI061093-059001/AI/NIAID NIH HHS/United States
- P01 AI061093-065495/AI/NIAID NIH HHS/United States
- T32 AI007621/AI/NIAID NIH HHS/United States
- P01 AI061093-04/AI/NIAID NIH HHS/United States
- P01 AI061093/AI/NIAID NIH HHS/United States
- R01 AI057653-02/AI/NIAID NIH HHS/United States
- P01 AI061093-065490/AI/NIAID NIH HHS/United States
- R01 AI074378/AI/NIAID NIH HHS/United States
- R01 AI057653-01A1/AI/NIAID NIH HHS/United States
- R01 AI074378-04/AI/NIAID NIH HHS/United States
- P01 AI061093-06/AI/NIAID NIH HHS/United States
- R01 AI057653-05/AI/NIAID NIH HHS/United States
- P01 AI061093-05/AI/NIAID NIH HHS/United States
- R01 AI074378-02/AI/NIAID NIH HHS/United States
- R01 AI057653/AI/NIAID NIH HHS/United States
- R01 AI057653-02S1/AI/NIAID NIH HHS/United States
- R01 AI074378-01A1/AI/NIAID NIH HHS/United States
- R01 AI074378-03/AI/NIAID NIH HHS/United States
- R01 AI057653-04/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources


