Pharmacokinetics of subcutaneous recombinant methionyl human leptin administration in healthy subjects in the fed and fasting states: regulation by gender and adiposity
- PMID: 18840030
- PMCID: PMC2737404
- DOI: 10.2165/00003088-200847110-00006
Pharmacokinetics of subcutaneous recombinant methionyl human leptin administration in healthy subjects in the fed and fasting states: regulation by gender and adiposity
Abstract
Background: Recombinant methionyl human leptin (r-metHuLeptin) has demonstrated efficacy in improving hormonal and metabolic parameters in leptin-deficient states, and it has been suggested that leptin replacement may reverse metabolic adaptations during weight loss interventions. The pharmacokinetics of subcutaneously administered r-metHuLeptin have been recently published, but whether pharmacokinetic parameters are altered by short-term fasting, adiposity and/or gender has not yet been evaluated.
Objective: The objective of this study was to characterize pharmacokinetic parameters following subcutaneous r-metHuLeptin administration at doses in the physiological to supra-physiological to pharmacological range in the fed state and during 3-day complete fasting in lean and obese subjects, including both men and women.
Methods: We analysed pharmacokinetic profiles in five lean men, five obese men and five lean women following subcutaneous administration of physiological (0.01 mg/kg), supra-physiological (0.1 mg/kg) and pharmacological (0.3 mg/kg) doses of r-metHuLeptin given once in the fed state and once daily during 3-day complete caloric deprivation (fasting).
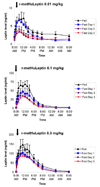
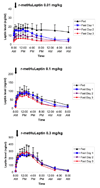
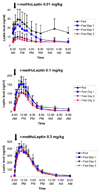
Results: With r-metHuLeptin administration at 0.01 mg/kg, leptin concentrations ranged up to approximately 7 ng/mL in lean men, approximately 20 ng/mL in obese men and approximately 30 ng/mL in lean women in the fed state. There was a significant effect of 3-day fasting: it decreased baseline leptin concentrations, peak serum concentration (C(max)) and area under the serum concentration-time curve from time zero to infinity (AUC(infinity)) [all p < 0.0001] and increased clearance (p < 0.001), most prominently in lean men (p < 0.0001 across the groups). Administration of r-metHuLeptin at 0.1 mg/kg resulted in leptin concentrations up to approximately 70 ng/mL in lean men, approximately 100 ng/mL in obese men and approximately 150 ng/mL in lean women in the fed state. At this dose, there was a similar effect of fasting on the pharmacokinetic parameters as well as a decrease in the terminal-phase elimination half-life (p = 0.02), consistent with increased clearance, but the effect of fasting was less pronounced overall than with the 0.01 mg/kg dose. With r-metHuLeptin administration at 0.3 mg/kg, leptin concentrations ranged up to approximately 150 ng/mL in lean men, approximately 300 ng/mL in obese men and approximately 400 ng/mL in lean women in the fed state. At this dose, fasting increased clearance to a lesser degree (p = 0.046), mainly in lean men, suggesting that the fasting-induced increase in leptin clearance by the kidneys can plateau. Within each group, the subjects lost approximately 3-4 kg of bodyweight after 3 days of fasting (all p < 0.0001), but the amount and time course of weight loss did not differ according to the dose of r-metHuLeptin administered or the circulating leptin concentrations achieved.
Conclusions: Short-term fasting in healthy individuals results in increased clearance of leptin; this contributes to hypoleptinaemia, which may serve as a signal to increase energy intake in the setting of caloric restriction. Obese individuals with greater energy stores at baseline have a blunted response to the fasting-induced increase in leptin clearance. Also, women have a differential response to fasting, with primarily decreased leptin production rather than increased clearance. These findings and the resulting formulas for calculating doses for r-metHuLeptin administration have important implications for future therapeutic use of r-metHuLeptin in conjunction with hypocaloric diets for the treatment of obesity.
Figures
Similar articles
-
Pharmacokinetics of recombinant methionyl human leptin after subcutaneous administration: variation of concentration-dependent parameters according to assay.J Clin Endocrinol Metab. 2007 Jun;92(6):2307-11. doi: 10.1210/jc.2006-2864. Epub 2007 Apr 3. J Clin Endocrinol Metab. 2007. PMID: 17405837 Clinical Trial.
-
Leptin hormonal kinetics in the fed state: effects of adiposity, age, and gender on endogenous leptin production and clearance rates.J Clin Endocrinol Metab. 2004 Jun;89(6):2672-7. doi: 10.1210/jc.2003-031931. J Clin Endocrinol Metab. 2004. PMID: 15181040 Clinical Trial.
-
Circulating melanin-concentrating hormone, agouti-related protein, and alpha-melanocyte-stimulating hormone levels in relation to body composition: alterations in response to food deprivation and recombinant human leptin administration.J Clin Endocrinol Metab. 2005 Feb;90(2):1047-54. doi: 10.1210/jc.2004-1124. Epub 2004 Nov 16. J Clin Endocrinol Metab. 2005. PMID: 15546902
-
Therapeutic use of recombinant methionyl human leptin.Biochimie. 2012 Oct;94(10):2116-25. doi: 10.1016/j.biochi.2012.03.013. Epub 2012 Mar 23. Biochimie. 2012. PMID: 22464954 Review.
-
Clinical aspects of leptin.Vitam Horm. 1998;54:1-30. doi: 10.1016/s0083-6729(08)60919-x. Vitam Horm. 1998. PMID: 9529971 Review.
Cited by
-
Total and H-specific GDF-15 levels increase in caloric deprivation independently of leptin in humans.Nat Commun. 2024 Jun 18;15(1):5190. doi: 10.1038/s41467-024-49366-y. Nat Commun. 2024. PMID: 38890300 Free PMC article.
-
Pharmacotherapy of obesity: an update on the available medications and drugs under investigation.EClinicalMedicine. 2023 Mar 20;58:101882. doi: 10.1016/j.eclinm.2023.101882. eCollection 2023 Apr. EClinicalMedicine. 2023. PMID: 36992862 Free PMC article. Review.
-
Does Food Affect the Pharmacokinetics of Non-orally Delivered Drugs? A Review of Currently Available Evidence.AAPS J. 2022 Apr 29;24(3):59. doi: 10.1208/s12248-022-00714-0. AAPS J. 2022. PMID: 35488003 Review.
-
Leptin in Leanness and Obesity: JACC State-of-the-Art Review.J Am Coll Cardiol. 2021 Feb 16;77(6):745-760. doi: 10.1016/j.jacc.2020.11.069. J Am Coll Cardiol. 2021. PMID: 33573745 Free PMC article. Review.
-
Leptin alters energy intake and fat mass but not energy expenditure in lean subjects.Nat Commun. 2020 Oct 13;11(1):5145. doi: 10.1038/s41467-020-18885-9. Nat Commun. 2020. PMID: 33051459 Free PMC article.
References
-
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 01;372(6505):425–432. - PubMed
-
- Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002 Feb 21;346(8):570–578. - PubMed
-
- Lee JH, Chan JL, Sourlas E, Raptopoulos V, Mantzoros CS. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006 Jul;91(7):2605–2611. - PubMed
-
- Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004 Sep 02;351(10):987–997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK079929-01A1/DK/NIDDK NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- R01-79929/PHS HHS/United States
- P30 DK040561-12/DK/NIDDK NIH HHS/United States
- K24 DK081913/DK/NIDDK NIH HHS/United States
- M01 RR001032/RR/NCRR NIH HHS/United States
- K24 81913/PHS HHS/United States
- M01-RR01032/RR/NCRR NIH HHS/United States
- R01 DK079929/DK/NIDDK NIH HHS/United States
- K23 RR018860-05/RR/NCRR NIH HHS/United States
- R01-58785/PHS HHS/United States
- K24 DK081913-01A1/DK/NIDDK NIH HHS/United States
- K23 RR018860/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources


