Structural basis for potent cross-neutralizing human monoclonal antibody protection against lethal human and zoonotic severe acute respiratory syndrome coronavirus challenge
- PMID: 18199635
- PMCID: PMC2268459
- DOI: 10.1128/JVI.02377-07
Structural basis for potent cross-neutralizing human monoclonal antibody protection against lethal human and zoonotic severe acute respiratory syndrome coronavirus challenge
Abstract
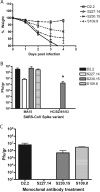
Severe acute respiratory syndrome coronavirus (SARS-CoV) emerged in 2002, and detailed phylogenetic and epidemiological analyses have suggested that it originated from animals. The spike (S) glycoprotein has been identified as a major component of protective immunity, and 23 different amino acid changes were noted during the expanding epidemic. Using a panel of SARS-CoV recombinants bearing the S glycoproteins from isolates representing the zoonotic and human early, middle, and late phases of the epidemic, we identified 23 monoclonal antibodies (MAbs) with neutralizing activity against one or multiple SARS-CoV spike variants and determined the presence of at least six distinct neutralizing profiles in the SARS-CoV S glycoprotein. Four of these MAbs showed cross-neutralizing activity against all human and zoonotic S variants in vitro, and at least three of these were mapped in distinct epitopes using escape mutants, structure analyses, and competition assays. These three MAbs (S109.8, S227.14, and S230.15) were tested for use in passive vaccination studies using lethal SARS-CoV challenge models for young and senescent mice with four different homologous and heterologous SARS-CoV S variants. Both S227.14 and S230.15 completely protected young and old mice from weight loss and virus replication in the lungs for all viruses tested, while S109.8 completely protected mice from weight loss and clinical signs in the presence of viral titers. We conclude that a single human MAb can confer broad protection against lethal challenge with multiple zoonotic and human SARS-CoV isolates, and we identify a robust cocktail formulation that targets distinct epitopes and minimizes the likely generation of escape mutants.
Figures
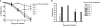
Similar articles
-
Development and characterization of a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice.J Infect Dis. 2005 Feb 15;191(4):507-14. doi: 10.1086/427242. Epub 2005 Jan 14. J Infect Dis. 2005. PMID: 15655773 Free PMC article.
-
Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants.PLoS Med. 2006 Jul;3(7):e237. doi: 10.1371/journal.pmed.0030237. PLoS Med. 2006. PMID: 16796401 Free PMC article.
-
Escape from human monoclonal antibody neutralization affects in vitro and in vivo fitness of severe acute respiratory syndrome coronavirus.J Infect Dis. 2010 Mar 15;201(6):946-55. doi: 10.1086/651022. J Infect Dis. 2010. PMID: 20144042 Free PMC article.
-
Structural Analysis of Neutralizing Epitopes of the SARS-CoV-2 Spike to Guide Therapy and Vaccine Design Strategies.Viruses. 2021 Jan 19;13(1):134. doi: 10.3390/v13010134. Viruses. 2021. PMID: 33477902 Free PMC article. Review.
-
Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential.Rev Med Virol. 2012 Jan;22(1):2-17. doi: 10.1002/rmv.706. Epub 2011 Sep 8. Rev Med Virol. 2012. PMID: 21905149 Free PMC article. Review.
Cited by
-
High transmission of endemic human coronaviruses before and during the COVID-19 pandemic in adolescents in Cebu, Philippines.BMC Infect Dis. 2024 Sep 27;24(1):1042. doi: 10.1186/s12879-024-09672-8. BMC Infect Dis. 2024. PMID: 39333882 Free PMC article.
-
Bispecific antibodies provide broad neutralization of emerging beta-coronaviruses by targeting ACE2 and viral spikes.Emerg Microbes Infect. 2024 Dec;13(1):2404166. doi: 10.1080/22221751.2024.2404166. Epub 2024 Sep 22. Emerg Microbes Infect. 2024. PMID: 39258934 Free PMC article.
-
Expression, Purification, and Evaluation of Antibody Responses and Antibody-Immunogen Complex Simulation of a Designed Multi-Epitope Vaccine against SARS-COV-2.Protein Pept Lett. 2024;31(8):619-638. doi: 10.2174/0109298665320319240809095727. Protein Pept Lett. 2024. PMID: 39162285
-
Preclinical safety and efficacy of a therapeutic antibody that targets SARS-CoV-2 at the sotrovimab face but is escaped by Omicron.iScience. 2023 Apr 21;26(4):106323. doi: 10.1016/j.isci.2023.106323. Epub 2023 Mar 2. iScience. 2023. PMID: 36925720 Free PMC article.
-
Nanoluciferase-based cell fusion assay for rapid and high-throughput assessment of SARS-CoV-2-neutralizing antibodies in patient samples.Methods Enzymol. 2022;675:351-381. doi: 10.1016/bs.mie.2022.07.015. Epub 2022 Sep 9. Methods Enzymol. 2022. PMID: 36220277 Free PMC article.
References
-
- Bakker, A. B., W. E. Marissen, R. A. Kramer, A. B. Rice, W. C. Weldon, M. Niezgoda, C. A. Hanlon, S. Thijsse, H. H. Backus, J. de Kruif, B. Dietzschold, C. E. Rupprecht, and J. Goudsmit. 2005. Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants. J. Virol. 799062-9068. - PMC - PubMed
-
- Chan, K. C., N. L. Tang, D. S. Hui, G. T. Chung, A. K. Wu, S. S. Chim, R. W. Chiu, N. Lee, K. W. Choi, Y. M. Sung, P. K. Chan, Y. K. Tong, S. T. Lai, W. C. Yu, O. Tsang, and Y. M. Lo. 2005. Absence of association between angiotensin converting enzyme polymorphism and development of adult respiratory distress syndrome in patients with severe acute respiratory syndrome: a case control study. BMC Infect. Dis. 526. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


