Escherichia coli ribosomal protein L20 binds as a single monomer to its own mRNA bearing two potential binding sites
- PMID: 17439971
- PMCID: PMC1888825
- DOI: 10.1093/nar/gkm197
Escherichia coli ribosomal protein L20 binds as a single monomer to its own mRNA bearing two potential binding sites
Abstract
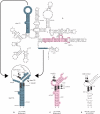
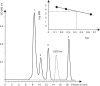
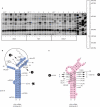
Ribosomal protein L20 is crucial for the assembly of the large ribosomal subunit and represses the translation of its own mRNA. L20 mRNA carries two L20-binding sites, the first folding into a pseudoknot and the second into an imperfect stem and loop. These two sites and the L20-binding site on 23S ribosomal RNA are recognized similarly using a single RNA-binding site located on one face of L20. In this work, using gel filtration and fluorescence cross-correlation spectroscopy (FCCS) experiments, we first exclude the possibility that L20 forms a dimer, which would allow each monomer to bind one site of the mRNA. Secondly we show, using affinity purification and FCCS experiments, that only one molecule of L20 binds to the L20 mRNA despite the presence of two potential binding sites. Thirdly, using RNA chemical probing, we show that the two L20-binding sites are in interaction. This interaction provides an explanation for the single occupancy of the mRNA. The two interacting sites could form a single hybrid site or the binding of L20 to a first site may inhibit binding to the second. Models of regulation compatible with our data are discussed.
Figures





Similar articles
-
Double molecular mimicry in Escherichia coli: binding of ribosomal protein L20 to its two sites in mRNA is similar to its binding to 23S rRNA.Mol Microbiol. 2005 Jun;56(6):1441-56. doi: 10.1111/j.1365-2958.2005.04644.x. Mol Microbiol. 2005. PMID: 15916597
-
A competition mechanism regulates the translation of the Escherichia coli operon encoding ribosomal proteins L35 and L20.J Mol Biol. 2008 Jan 18;375(3):612-25. doi: 10.1016/j.jmb.2007.10.058. Epub 2007 Nov 1. J Mol Biol. 2008. PMID: 18037435
-
Translational feedback regulation of the gene for L35 in Escherichia coli requires binding of ribosomal protein L20 to two sites in its leader mRNA: a possible case of ribosomal RNA-messenger RNA molecular mimicry.RNA. 2002 Jul;8(7):878-89. doi: 10.1017/s1355838202029084. RNA. 2002. PMID: 12166643 Free PMC article.
-
[Interactions of ribosomal protein L1 with ribosomal and messenger RNAs].Mol Biol (Mosk). 2006 Jul-Aug;40(4):650-7. Mol Biol (Mosk). 2006. PMID: 16913224 Review. Russian.
-
Hfq structure, function and ligand binding.Curr Opin Microbiol. 2007 Apr;10(2):125-33. doi: 10.1016/j.mib.2007.03.015. Epub 2007 Mar 28. Curr Opin Microbiol. 2007. PMID: 17395525 Review.
Cited by
-
Probing ribosomal protein-RNA interactions with an external force.Proc Natl Acad Sci U S A. 2011 Nov 8;108(45):18272-6. doi: 10.1073/pnas.1107121108. Epub 2011 Oct 24. Proc Natl Acad Sci U S A. 2011. PMID: 22025688 Free PMC article.
-
Regulation of translation initiation by RNA binding proteins.Annu Rev Microbiol. 2009;63:27-44. doi: 10.1146/annurev.micro.091208.073514. Annu Rev Microbiol. 2009. PMID: 19385727 Free PMC article. Review.
-
Discovery of functional elements in 12 Drosophila genomes using evolutionary signatures.Nature. 2007 Nov 8;450(7167):219-32. doi: 10.1038/nature06340. Nature. 2007. PMID: 17994088 Free PMC article.
References
-
- Romby P., Springer M. Translational control in prokaryotes. In: Matthews M.B., Sonenberg N., Hershey J.W.B., editors. Translational Control of Gene Expression. Cols Spring Harbor, Newyork: Cold Spring Harbor Laboratory Press; 2006.
-
- Hattman S. Unusual transcriptional and translational regulation of the bacteriophage Mu mom operon. Pharmacol. Ther. 1999;84:367–388. - PubMed
-
- Oppenheim A.B., Kornitzer D., Altuvia S., Court D.L. Posttranscriptional control of the lysogenic pathway in bacteriophage lambda. Prog. Nucleic Acid Res., Mol. Biol. 1993;46:37–49. - PubMed
-
- Zengel J.M., Lindahl L. Diverse mechanisms for regulating ribosomal protein synthesis in Escherichia coli. Prog. Nucleic Acid Res. Mol. Biol. 1994;47:331–370. - PubMed
-
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu. Rev. Biochem. 1984;53:75–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases


