Six1 and Eya1 expression can reprogram adult muscle from the slow-twitch phenotype into the fast-twitch phenotype
- PMID: 15226428
- PMCID: PMC434262
- DOI: 10.1128/MCB.24.14.6253-6267.2004
Six1 and Eya1 expression can reprogram adult muscle from the slow-twitch phenotype into the fast-twitch phenotype
Abstract
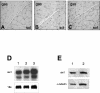
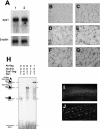
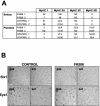
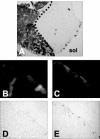
Muscle fibers show great differences in their contractile and metabolic properties. This diversity enables skeletal muscles to fulfill and adapt to different tasks. In this report, we show that the Six/Eya pathway is implicated in the establishment and maintenance of the fast-twitch skeletal muscle phenotype. We demonstrate that the MEF3/Six DNA binding element present in the aldolase A pM promoter mediates the high level of activation of this promoter in fast-twitch glycolytic (but not in slow-twitch) muscle fibers. We also show that among the Six and Eya gene products expressed in mouse skeletal muscle, Six1 and Eya1 proteins accumulate preferentially in the nuclei of fast-twitch muscles. The forced expression of Six1 and Eya1 together in the slow-twitch soleus muscle induced a fiber-type transition characterized by the replacement of myosin heavy chain I and IIA isoforms by the faster IIB and/or IIX isoforms, the activation of fast-twitch fiber-specific genes, and a switch toward glycolytic metabolism. Collectively, these data identify Six1 and Eya1 as the first transcriptional complex that is able to reprogram adult slow-twitch oxidative fibers toward a fast-twitch glycolytic phenotype.
Figures






Similar articles
-
[New insights into adult muscle fiber-type diversity: involvement of Six homeoproteins].Bull Acad Natl Med. 2015 Jan;199(1):21-31. Bull Acad Natl Med. 2015. PMID: 27236875 French.
-
The homoeobox gene SIX1 alters myosin heavy chain isoform expression in mouse skeletal muscle.Acta Physiol (Oxf). 2014 Feb;210(2):415-28. doi: 10.1111/apha.12168. Epub 2013 Nov 11. Acta Physiol (Oxf). 2014. PMID: 24102895
-
TnIfast IRE enhancer: multistep developmental regulation during skeletal muscle fiber type differentiation.Dev Dyn. 2002 Aug;224(4):422-31. doi: 10.1002/dvdy.10122. Dev Dyn. 2002. PMID: 12203734
-
Muscle mechanics: adaptations with exercise-training.Exerc Sport Sci Rev. 1996;24:427-73. Exerc Sport Sci Rev. 1996. PMID: 8744258 Review.
-
The adaptive potential of skeletal muscle fibers.Can J Appl Physiol. 2002 Aug;27(4):423-48. doi: 10.1139/h02-023. Can J Appl Physiol. 2002. PMID: 12442355 Review.
Cited by
-
DiPRO1 distinctly reprograms muscle and mesenchymal cancer cells.EMBO Mol Med. 2024 Aug;16(8):1840-1885. doi: 10.1038/s44321-024-00097-z. Epub 2024 Jul 15. EMBO Mol Med. 2024. PMID: 39009887 Free PMC article.
-
Whole genome sequencing of a family with autosomal dominant features within the oculoauriculovertebral spectrum.medRxiv [Preprint]. 2024 Jul 15:2024.02.07.24301824. doi: 10.1101/2024.02.07.24301824. medRxiv. 2024. PMID: 38370836 Free PMC article. Preprint.
-
Molecular mechanisms underlying the impact of muscle fiber types on meat quality in livestock and poultry.Front Vet Sci. 2023 Nov 22;10:1284551. doi: 10.3389/fvets.2023.1284551. eCollection 2023. Front Vet Sci. 2023. PMID: 38076559 Free PMC article. Review.
-
Phenotypic and molecular basis of SIX1 variants linked to non-syndromic deafness and atypical branchio-otic syndrome in South Korea.Sci Rep. 2023 Jul 21;13(1):11776. doi: 10.1038/s41598-023-38909-w. Sci Rep. 2023. PMID: 37479820 Free PMC article.
-
Developmental, physiologic and phylogenetic perspectives on the expression and regulation of myosin heavy chains in mammalian skeletal muscles.J Comp Physiol B. 2023 Aug;193(4):355-382. doi: 10.1007/s00360-023-01499-0. Epub 2023 Jun 5. J Comp Physiol B. 2023. PMID: 37277594 Free PMC article. Review.
References
-
- Abdelhak, S., V. Kalatzis, R. Heilig, S. Compain, D. Samson, C. Vincent, F. Levi-Acobas, C. Cruaud, M. Le Merrer, M. Mathieu, R. Konig, J. Vigneron, J. Weissenbach, C. Petit, and D. Weil. 1997. Clustering of mutations responsible for branchio-oto-renal (BOR) syndrome in the eyes absent homologous region (eyaHR) of EYA1. Hum. Mol. Genet. 6:2247-2255. - PubMed
-
- Anakwe, K., L. Robson, J. Hadley, P. Buxton, S. Allen, C. Hartmann, B. Harfe, T. Nohno, D. Evans, and P. Francis-West. 2003. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development 130:3503-3515. - PubMed
-
- Bertrand, A., V. Ngo-Muller, D. Hentzen, J.-P. Concordet, D. Daegelen, and D. Tuil. 2003. Muscle electrotransfer as a tool for studying muscle fiber-specific and nerve-dependent activity of promoters. Am. J. Physiol. Cell Physiol. 285:C1071-C1081. - PubMed
-
- Borsani, G., A. DeGrandi, A. Ballabio, A. B. Bulfone, L. Bernard, S. Banfi, C. Gattuso, M. Mariani, M. Dixon, D. Donnai, K. Metcalfe, R. Winter, M. Robertson, R. Axton, A. Brown, V. van Heyningen, and I. M. Hanson. 1999. EYA4, a novel vertebrate gene related to Drosophila eyes absent. Hum. Mol. Genet. 8:11-23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

