In vivo detection and quantification of tetracycline by use of a whole-cell biosensor in the rat intestine
- PMID: 15047509
- PMCID: PMC375317
- DOI: 10.1128/AAC.48.4.1112-1117.2004
In vivo detection and quantification of tetracycline by use of a whole-cell biosensor in the rat intestine
Abstract
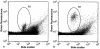
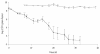
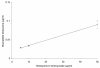
An Escherichia coli biosensor strain, harboring the plasmid pTGFP2, was introduced into the gastrointestinal tract of gnotobiotic rats that continuously received drinking water containing tetracycline. Plasmid pTGFP2 contains a transcriptional fusion between a green fluorescent protein (GFP) gene and a tetracycline-regulated promoter and was shown to produce a proportional GFP signal in response to exposure to various tetracycline concentrations when harbored by an E. coli strain. The plasmid was highly unstable in the host bacteria colonizing the intestinal system of the animals, and rapid plasmid loss was observed. Reintroduction of the E. coli MC4100/pTGFP2 strain into animals already colonized by the plasmid-free E. coli strain the day before euthanasia made it possible to extract and analyze the biosensors from intestinal samples. The induction of GFP in the biosensor cells extracted from the animals was estimated on a single-cell basis by use of flow cytometry, and the mean induction of GFP in the samples was compared to a standard curve prepared from known tetracycline concentrations. The results showed that the bioavailable tetracycline concentration within the bacterial growth habitat of the intestine was proportional to the concentration of tetracycline in drinking water but represented only approximately 0.4% of the intake concentration. This is a significant finding which will help to clarify antimicrobial therapy in the intestinal environment.
Figures

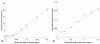
Similar articles
-
Construction of an extended range whole-cell tetracycline biosensor by use of the tet(M) resistance gene.FEMS Microbiol Lett. 2005 Dec 15;253(2):201-5. doi: 10.1016/j.femsle.2005.09.034. Epub 2005 Oct 7. FEMS Microbiol Lett. 2005. PMID: 16239081
-
Quantification of bioavailable chlortetracycline in pig feces using a bacterial whole-cell biosensor.Vet Microbiol. 2002 Jun 5;87(1):51-7. doi: 10.1016/s0378-1135(02)00029-9. Vet Microbiol. 2002. PMID: 12079746
-
Presence of N-acyl homoserine lactones in soil detected by a whole-cell biosensor and flow cytometry.Microb Ecol. 2003 Mar;45(3):226-36. doi: 10.1007/s00248-002-2028-6. Epub 2003 Mar 28. Microb Ecol. 2003. PMID: 12658522
-
Effect of tetracycline on transfer and establishment of the tetracycline-inducible conjugative transposon Tn916 in the guts of gnotobiotic rats.Appl Environ Microbiol. 2004 Feb;70(2):758-64. doi: 10.1128/AEM.70.2.758-764.2004. Appl Environ Microbiol. 2004. PMID: 14766552 Free PMC article.
-
Green fluorescent protein for detection of the probiotic microorganism Escherichia coli strain Nissle 1917 (EcN) in vivo.J Microbiol Methods. 2005 Jun;61(3):389-98. doi: 10.1016/j.mimet.2005.01.007. J Microbiol Methods. 2005. PMID: 15767015
Cited by
-
Status quo of tet regulation in bacteria.Microb Biotechnol. 2022 Apr;15(4):1101-1119. doi: 10.1111/1751-7915.13926. Epub 2021 Oct 29. Microb Biotechnol. 2022. PMID: 34713957 Free PMC article. Review.
-
Monte Carlo Simulations Suggest Current Chlortetracycline Drug-Residue Based Withdrawal Periods Would Not Control Antimicrobial Resistance Dissemination from Feedlot to Slaughterhouse.Front Microbiol. 2017 Sep 20;8:1753. doi: 10.3389/fmicb.2017.01753. eCollection 2017. Front Microbiol. 2017. PMID: 29033901 Free PMC article.
-
Models of antimicrobial pressure on intestinal bacteria of the treated host populations.Epidemiol Infect. 2017 Jul;145(10):2081-2094. doi: 10.1017/S095026881700084X. Epub 2017 May 2. Epidemiol Infect. 2017. PMID: 28462738 Free PMC article.
-
The application of Tet repressor in prokaryotic gene regulation and expression.Microb Biotechnol. 2008 Jan;1(1):2-16. doi: 10.1111/j.1751-7915.2007.00001.x. Microb Biotechnol. 2008. PMID: 21261817 Free PMC article. Review.
-
Applications of flow cytometry in environmental microbiology and biotechnology.Extremophiles. 2009 May;13(3):389-401. doi: 10.1007/s00792-009-0236-4. Epub 2009 Mar 20. Extremophiles. 2009. PMID: 19301090 Review.
References
-
- Blake, D. P., R. W. Humphry, K. P. Scott, K. Hillman, D. R. Fenlon, and J. C. Low. 2003. Influence of tetracycline exposure on tetracycline resistance and the carriage of tetracycline resistance genes within commensal Escherichia coli populations. J. Appl. Microbiol. 94:1087-1097. - PubMed
-
- Celli, J., and P. Trieu-Cuot. 1998. Circularization of Tn916 is required for expression of the transposon-encoded transfer functions: characterization of long tetracycline-inducible transcripts reading through the attachment site. Mol. Microbiol. 28:103-117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


