Inhibition of huntingtin fibrillogenesis by specific antibodies and small molecules: implications for Huntington's disease therapy
- PMID: 10829068
- PMCID: PMC18723
- DOI: 10.1073/pnas.110138997
Inhibition of huntingtin fibrillogenesis by specific antibodies and small molecules: implications for Huntington's disease therapy
Abstract
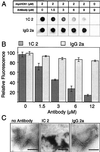
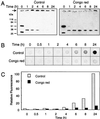
The accumulation of insoluble protein aggregates in intra and perinuclear inclusions is a hallmark of Huntington's disease (HD) and related glutamine-repeat disorders. A central question is whether protein aggregation plays a direct role in the pathogenesis of these neurodegenerative diseases. Here we show by using a filter retardation assay that the mAb 1C2, which specifically recognizes the elongated polyglutamine (polyQ) stretch in huntingtin, and the chemical compounds Congo red, thioflavine S, chrysamine G, and Direct fast yellow inhibit HD exon 1 protein aggregation in a dose-dependent manner. On the other hand, potential inhibitors of amyloid-beta formation such as thioflavine T, gossypol, melatonin, and rifampicin had little or no inhibitory effect on huntingtin aggregation in vitro. The results obtained by the filtration assay were confirmed by electron microscopy, SDS/PAGE, and MS. Furthermore, cell culture studies revealed that the Congo red dye at micromolar concentrations reduced the extent of HD exon 1 aggregation in transiently transfected COS cells. Together, these findings contribute to a better understanding of the mechanism of huntingtin fibrillogenesis in vitro and provide the basis for the development of new huntingtin aggregation inhibitors that may be effective in treating HD.
Figures




Similar articles
-
Identification of benzothiazoles as potential polyglutamine aggregation inhibitors of Huntington's disease by using an automated filter retardation assay.Proc Natl Acad Sci U S A. 2002 Dec 10;99 Suppl 4(Suppl 4):16400-6. doi: 10.1073/pnas.182426599. Epub 2002 Aug 28. Proc Natl Acad Sci U S A. 2002. PMID: 12200548 Free PMC article.
-
Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington's disease.Proc Natl Acad Sci U S A. 2001 Apr 10;98(8):4764-9. doi: 10.1073/pnas.071058398. Proc Natl Acad Sci U S A. 2001. PMID: 11296304 Free PMC article.
-
Reversal of a full-length mutant huntingtin neuronal cell phenotype by chemical inhibitors of polyglutamine-mediated aggregation.BMC Neurosci. 2005 Jan 13;6:1. doi: 10.1186/1471-2202-6-1. BMC Neurosci. 2005. PMID: 15649316 Free PMC article.
-
[Huntington disease. A review].Invest Clin. 2000 Jun;41(2):117-41. Invest Clin. 2000. PMID: 10961047 Review. Spanish.
-
Challenges of Huntington's disease and quest for therapeutic biomarkers.Proteomics Clin Appl. 2015 Feb;9(1-2):147-58. doi: 10.1002/prca.201400073. Epub 2014 Nov 2. Proteomics Clin Appl. 2015. PMID: 25290828 Review.
Cited by
-
Small Molecules Inducing Autophagic Degradation of Expanded Polyglutamine Protein through Interaction with Both Mutant ATXN3 and LC3.Int J Mol Sci. 2024 Oct 4;25(19):10707. doi: 10.3390/ijms251910707. Int J Mol Sci. 2024. PMID: 39409036 Free PMC article.
-
Why Is Arginine the Only Amino Acid That Inhibits Polyglutamine Monomers from Taking on Toxic Conformations?ACS Chem Neurosci. 2024 Aug 7;15(15):2925-2935. doi: 10.1021/acschemneuro.4c00276. Epub 2024 Jul 15. ACS Chem Neurosci. 2024. PMID: 39009034 Free PMC article.
-
Highly Potent Peptide Therapeutics To Prevent Protein Aggregation in Huntington's Disease.ACS Med Chem Lett. 2023 Nov 14;14(12):1821-1826. doi: 10.1021/acsmedchemlett.3c00415. eCollection 2023 Dec 14. ACS Med Chem Lett. 2023. PMID: 38116434
-
A Novel Huntington's Disease Assessment Platform to Support Future Drug Discovery and Development.Int J Mol Sci. 2022 Nov 25;23(23):14763. doi: 10.3390/ijms232314763. Int J Mol Sci. 2022. PMID: 36499090 Free PMC article.
-
Non-Cell Autonomous and Epigenetic Mechanisms of Huntington's Disease.Int J Mol Sci. 2021 Nov 19;22(22):12499. doi: 10.3390/ijms222212499. Int J Mol Sci. 2021. PMID: 34830381 Free PMC article. Review.
References
-
- Harper P S. Huntington's Disease. Philadelphia: Saunders; 1991.
-
- Vonsattel J-P, Meyers R H, Stevens T J, Ferrante R J, Bird E D, Richardson E P. J Neuropathol Exp Neurol. 1985;44:559–577. - PubMed
-
- Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, Bates G P, Davies S W, Lehrach H, Wanker E E. Cell. 1997;90:549–558. - PubMed
-
- Davies S W, Trumaine M, Cozens B A, DiFiglia M, Sharp A H, Ross C A, Scherzinger E, Wanker E E, Mangiarini L, Bates G P. Cell. 1997;90:537–548. - PubMed
-
- Warrick J M, Paulson H L, Gray-Board G L, Bui Q T, Fischbeck K H, Pittman R N, Bonini N M. Cell. 1998;93:939–949. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


