Abstract
Study Objectives:
Menarche is a critical milestone in a woman’s life, and historically has been determined through several approaches. The goals of this study were to: 1) determine age at menarche from multiple reports of parents and adolescent participants in a prospective study; 2) examine factors impacting age at menarche; and 3) determine correlates of menarche and pubertal tempo.
Design:
Longitudinal observational study.
Setting:
Three sites of the Breast Cancer and the Environment Research Program.
Participants:
Girls enrolled at 6-8 years of age.
Main outcome measures:
Parental and participant reported age of menarche, tempo of puberty.
Results:
There were 946 girls who were assigned an age of menarche. The correlation between parent and participant reports was high (Spearman R= 0.799, p< 0.001), and the difference was insignificant. Median age at menarche overall was 12.25 years. Compared to Black participants, Hispanic girls were more likely to have menarche earlier, while White and Asian girls were more likely to have menarche later. Age of menarche was highly correlated with age of breast development (Spearman R= 0.547, p< .001), and inversely with BMI (Spearman R= −0.403, p< .001). Tempo (interval of age of breast development to menarche) was slower in those with earlier breast development.
Conclusions:
Parental and adolescent reports of menarche are highly correlated. Earlier breast maturation was associated with slower tempo through puberty. BMI had a greater impact on age at menarche than did race and ethnicity.
Keywords: menarche, puberty, pubertal tempo, breast development
Introduction
Menarche is a critical milestone in a woman’s life, from both socio-cultural and medical perspectives. Age at menarche has been extensively studied and identified as a risk factor for many health outcomes in adolescence and adulthood, as relative timing of maturation impacts engagement in risky behaviors during adolescence,1,2 breast cancer risk,3 and all-causes mortality. Accurate assignment of menarcheal age is critical for epidemiologic studies. In epidemiologic analyses, it common to assign age at menarche from a single recalled date or age with a precision of approximately one half year.4
The Breast Cancer and the Environment Research Program (BCERP) puberty cohort was established to examine factors that influence onset of pubertal maturation, in recognition of puberty as a window of susceptibility for development of breast cancer.
The goals of this current study were to: 1) determine age at menarche from multiple reports of parents and adolescent participants in a prospective study; 2) examine factors related to age at menarche; and 3) determine correlates of menarche and pubertal tempo.
Methods
Study Population:
The puberty studies of the Breast Cancer and the Environment Research Program are three prospective cohorts of 1257 young girls, enrolled at 6-8 years of age, 2004 to 2007, and followed up at least annually through 2014. Study sites were at Mount Sinai Medical Center in East Harlem, New York (NY), Cincinnati Children’s Hospital Medical Center (OH) and Kaiser Permanente of Northern California (CA). The main goal of the puberty studies was to investigate the role of the environment on pubertal development.5 Information was collected in the preferred language (English/Spanish) of the parent/guardian. Prior to data collection, informed consent was obtained from all parents/guardians and assent was obtained from the girl. The study protocols were approved by each site’s institutional review board.
Age at menarche ascertainment:
Beginning in the second year (NY and CA, ages 7-9 years) or third year (OH, ages 8-10 years) of follow-up interviews, parents or legal guardians provided information annually on their daughter’s first menstrual period. The parent was asked whether the girl had had her first menstrual period and, if she had, the month and year (or age, if no date was given). Information availability differed by study site, due to the combination of early loss-to-follow-up (NY and OH had the majority of loss by the first follow-up visit) and the later administration of menarche-related questions (first follow-up visit in NY/CA and second year of follow-up in OH). Beginning in the sixth year of follow-up, the girls at all sites were also asked whether they had had a period and in what month and year (or age). Girls who had been asked at least once about menarche, and had complete demographic data, were included (N=1088), as others were lost to follow-up before menarche questions were asked.
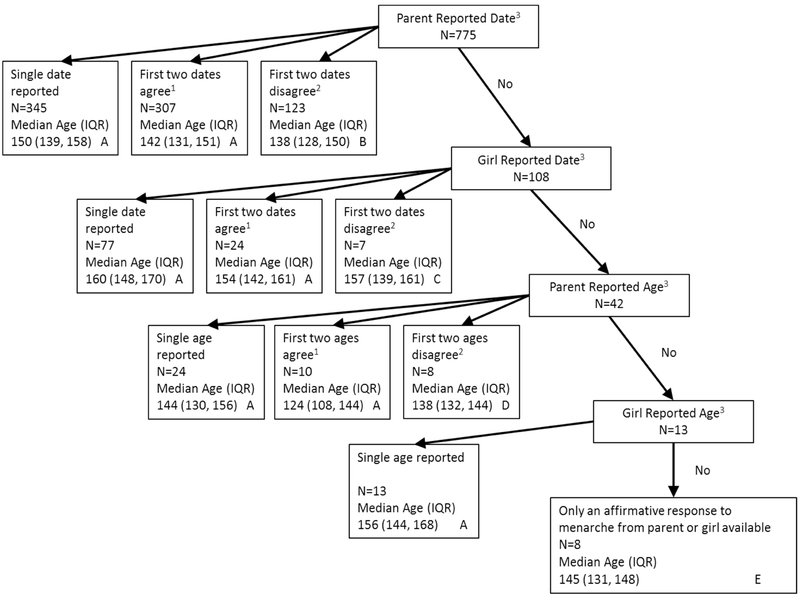
The age at menarche assignment algorithm used for this investigation is illustrated in Figure 1 for 946 girls who had complete demographic data and reported having reached menarche. Because parental/guardian menarcherelated information was available on the vast majority of girls and, in most cases, this information was provided closer in time to the menarche event, parent information was used as the primary source of information for age at menarche assignment; parent-provided information was used for assigning age at menarche for 822 girls (parent reported date (N=775) or age (N=42) or an affirmative answer without age or date (N=5)). Girls’ dates or ages were used for 124 assignments. As many as 8 parental reports and 5 self-reports were available for each girl. We used an actual reported value rather than an average of multiple reports. Because misinformation potentially increases with longer intervals between the event and report, the first two reports were chosen as the primary information for assignment of age at menarche. We considered dates to be more precise (± 1 month) than age (± 3-6 months), and preferentially used dates if available; for example, a girl’s report of a date superseded a parental age report (Figure 1). When reported dates were used for assignment of age at menarche and the difference between the first two reports was less than six months, then the first report was used. If the difference was six months or more, and if a third report was available, age at menarche was assigned using the reported date closest to the third reported date. Otherwise the first of the two reported dates was used. When only one report was available or only two reports greater than six months apart with no third report available, the first report was used for age at menarche assignment. This same procedure was implemented when reported age was used for assignment of age at menarche. If the only available information was an affirmative answer to the menstrual screening question, but not age or date, then age at menarche was imputed as 9 months prior to the age at interview when the answer was provided. Nine months was chosen because it was the midpoint of the median interval between the age at the last interview before a menarche report, and the determined age at menarche among girls with complete information
Figure 1:
Flow chart of the age at menarche assignment algorithm for 946 girls with an ascertained age. Median and IQR were computed from the survival distribution using SAS Proc PHREG with an additional strata statement adjusting for site.
1. Agree = difference within 6 months.
2. Disagree = difference 6+ months.
3. At least one report.
A. First reported date/age was used to determine age at menarche.
B. The first girl reported date was used as a tie breaker. If the first girl reported date was within 6 months of the first parent reported date, then first parent reported date was used. If the first girl reported date was within 6 months of the second parent reported date, then the second parent reported date was used. If there is no agreement among all dates, the first parent reported date was used.
C. The third girl reported date was used as a tie breaker. If the third girl reported date was within 6 months of the first girl reported date, then the first girl reported date was used. If the third girl reported date was within 6 months of the second girl reported date, then the second girl reported date was used. If there is no agreement among all dates, the first girl reported date was used.
D. Third parent reported age was used as a tie breaker. If the third parent reported age was within 6 months of the first parent reported age, then the first parent reported age was used. If the third parent reported age was within 6 months of the second parent reported age, the second parent reported age was used. If there is no agreement among all ages, the first parent reported age was used.
E. Age at menarche was computed as nine months prior to first affirmative response to menarche.
Covariates:
Information on a wide range of covariates was ascertained from the girl’s parent/guardian, including demographic data (race, ethnicity, annual income, parent education). A physical exam was performed on the girls at each visit during using standardized protocols to assess pubertal development and anthropometric measures including height and weight. Details of these protocols have been published previously.5 Body Mass Index (BMI) was calculated as weight in kg/height in cm2. Age- and sex-specific BMI percentile was estimated based on the 2000 growth charts from the Centers for Disease Control and Prevention (www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm).
Breast stage and tempo:
Pubertal maturation staging of both breast development and pubic hair appearance was performed by a health care professional or trained research personnel using a standardized protocol.5 Age at first breast development (breast stage 2, B2) was assigned as the age at the midpoint of the interval between the interview at the last visit at which breast stage 1 was observed and the first interview at which B2 or higher was observed.6 Tempo, defined as the interval between the start of breast development and menarche, was calculated as the difference between age at B2 and age at menarche. The tempo analyses included girls who had an age for both B2 and menarche, with tempo ≥ 0 (N=906).
Statistical Analysis:
Cox proportional hazard models were used to calculate hazard ratios and 95% confidence intervals to estimate the likelihood of earlier menarche associated with demographic characteristics and body size. With the availability of multiple BMI% measurements, BMI% was included as a time-dependent covariate at irregular time intervals. The median age at menarche within each demographic characteristics or body size category was computed from the survival distribution using Proc PHREG (SAS version 9.4) by specifying the strata and baseline statement. Because the baseline Proc PHREG statement does not support time-dependent variables, baseline BMI% was used to estimate median ages. Girls who did not reach menarche (n=143/1088) were included in the analysis as right-censored observations.
Linear regression models were used to evaluate tempo associations with demographic characteristics and body size category. Regression models were further stratified by age at B2 in order to address the concept that menarche has a ceiling age while B2 may start earlier or later. In other words, tempo could be the same length but could have different risk factors depending whether B2 was early or late (<9.3 or ≥ 9.3 years, median). In addition, if B2 were early, a wider range of tempo lengths is detectable than if B2 were later. In order to optimize power to study the B2-age by BMI% interaction in relation to tempo, we created a categorical joint variable with eight levels, dichotomous B2-age and four-level BMI% (normal, underweight, overweight, obese). The beta and 95% confidence interval from linear models can be interpreted as months of change in tempo length per unit of dependent variable.
Results
Table 1 presents study population characteristics for 1257 girls with or without menarche information. There were 946 girls who were assigned an age of menarche (median 12.2 years). For the majority of girls (86%), age at menarche was assigned based on parent’s report of first period (median 12.1 years). More assignments used parent than girl report because only parents provided information during the first five years of the study (see Figure 1, parent-reported date or age, n=775+42). Girls’ self-report (date or age) was used for 124 girls, median 13.1 years (see figure 1, girl self-reported date or age, n=108+13+3). These girls did not have an age reported by the parent. Data collection from the girls occurred only in the last four years of the study. Thus, any age at menarche derived from a girl’s report generally occurred when the girl was older, resulting in an older median age at menarche for this type of assignment.
Table 1:
Population characteristics by information provided on menarche
| Menarche InformationA |
No Menarche InformationB |
Overall | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | ||
| Race/ethnicity | Black | 338 | 84.5 | 62 | 15.5 | 400 |
| Hispanic | 334 | 88.1 | 45 | 11.9 | 379 | |
| Asian | 55 | 96.5 | 2 | 3.5 | 57 | |
| White | 361 | 85.8 | 60 | 14.3 | 421 | |
| SiteC | NY | 366 | 84.3 | 68 | 15.7 | 434 |
| OH | 291 | 76.8 | 88 | 23.2 | 379 | |
| CA | 431 | 97.1 | 13 | 2.9 | 444 | |
| Caregiver Education | <=High School | 314 | 87.7 | 44 | 12.3 | 358 |
| > High School | 741 | 88.4 | 97 | 11.6 | 838 | |
| Missing | 33 | 54.1 | 28 | 45.9 | 61 | |
| Age at Baseline | 6-6.9 years old | 349 | 85.1 | 61 | 14.9 | 410 |
| 7-7.9 years old | 578 | 87.2 | 85 | 12.8 | 663 | |
| 8+ | 161 | 87.5 | 23 | 12.5 | 184 | |
| Baseline BMI% | <50th | 363 | 87.7 | 51 | 12.3 | 414 |
| 50th-84.9th | 372 | 87.5 | 53 | 12.5 | 425 | |
| >85th | 352 | 84.8 | 63 | 15.2 | 415 | |
| Missing | 1 | 33.3 | 2 | 66.7 | 3 | |
| ALL | 1088 | 86.6 | 169 | 13.4 | 1257 | |
At least one question pertaining to menarche was completed. This group contains both girls who reported menarche and those who are right censored as of the 2012-2013 study year. This is the population used in analyses.
No questions pertaining to menarche were completed, not included further.
Chi-Square p-value < 0.05 for no information on menarche vs. available menarche information within the characteristic category.
When ages were available from both girl and parent reports during later study visits (N=359 pairs), the correlation between these two sources was high (Spearman R= 0.799, p < 0.001). Furthermore, the assigned ages were almost identical: median parent reported age: 11.6 years (IQR 10.9-12.4), median girl reported age: 11.7 (IQR 11.0-12.4), median paired difference: zero (data not shown). There was little difference in those parents with repeated reports (N=152, mean difference 0.42 years) and girls with repeated reports (N=275, mean difference 0.40 years).
Median age at menarche overall was 12.25 years. Age at menarche varied by race/ethnicity, site and body size (Table 1). Compared to Black girls, Hispanic girls were more likely to have menarche earlier, while White and Asian girls were more likely to have their first period later. These results were robust and remained significant (in White girls) or borderline significant (in Asian girls) after adjustment for study site and baseline body size. The apparent difference in age at menarche by study site did not remain after adjustment for race/ethnicity and body size. A very clear linear response was observed for increasing baseline body size with younger age at menarche, although adjustment for other covariates weakened the trend. Compared to girls who were normal weight at baseline (BMI% between 50th and 84.9th percentile), girls who were overweight or obese at baseline reached menarche 0.3 years earlier, while girls who were underweight at baseline reached menarche 0.5 years later (adjusted medians, Table 2). Moreover, the trajectories of BMI% with regard to menarche differed markedly by body size (data available). While BMI percentile among overweight girls remained fairly constant throughout puberty and post-menarche (approximately 90th percentile), it rose among normal and underweight girls during this interval.
Table 2:
Unadjusted and mutually adjusted median age at menarche and hazard ratios (HR) for age at menarche by study characteristics.
| Characteristic | N | Obs | Censored | Unadjusted | Mutually Adjusted | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median Menarche, yrA |
HR and 95%CIB | P value | Median Menarche, yrA |
HR and 95%CIB | P value | |||||
| Overall | 1088 | 945 | 143 | 12.25 | ||||||
| Race/Ethnicity | Black | 338 | 293 | 45 | 12.0 | ref | 11.83 | ref | ||
| Hispanic | 334 | 284 | 50 | 11.83 | 1.18 ( 1.00 - 1.39 ) | 0.04 | 11.58 | 1.15 ( 0.97 - 1.36 ) | 0.10 | |
| Asian | 55 | 53 | 2 | 12.75 | 0.67 ( 0.50 - 0.90 ) | <0.01 | 12.0 | 0.85 ( 0.62 - 1.17 ) | 0.32 | |
| White | 361 | 315 | 46 | 12.67 | 0.62 ( 0.53 - 0.72 ) | <0.0001 | 12.50 | 0.70 ( 0.58 - 0.84 ) | <0.0001 | |
| Site | NY | 366 | 298 | 68 | 11.83 | ref | 12.0 | ref | ||
| OH | 291 | 261 | 30 | 12.42 | 0.67 ( 0.57 - 0.80 ) | <0.0001 | 12.25 | 1.01 ( 0.83 - 1.23 ) | 0.92 | |
| CA | 431 | 386 | 45 | 12.5 | 0.67 ( 0.57 - 0.78 ) | <0.0001 | 12.42 | 0.87 ( 0.73 - 1.04 ) | 0.12 | |
| BMI%C | <50th | 363 | 311 | 52 | 12.83 | 0.54 ( 0.46 - 0.64 ) | <0.0001 | 13.08 | 0.54 ( 0.46 - 0.64 ) | <0.0001 |
| 50th-84.9th | 372 | 324 | 48 | 12.25 | ref | 12.58 | ref | |||
| 85th-94.9th | 172 | 148 | 24 | 11.92 | 1.30 ( 1.07 - 1.56 ) | <0.01 | 12.25 | 1.20 ( 0.99 - 1.45 ) | 0.06 | |
| >95th | 180 | 161 | 19 | 11.67 | 1.41 ( 1.17 - 1.70 ) | <0.001 | 12.25 | 1.20 ( 0.99 - 1.46 ) | 0.07 | |
Computed from the survival distribution within each characteristic using SAS Proc PHREG with an additional strata statement. The reference groups were Black, MSSM and BMI% 50th-84.5th. Baseline BMI% groupings were used to estimate medians.
Hazard ratios and 95% CIs were computed from Cox proportional hazard model using SAS Proc PHREG. BMI% was included as a time-varying variable.
BMI% as determined at baseline.
Age at menarche was highly correlated with age of B2 (Spearman R = 0.547, p < .001) and inversely with BMI (Spearman R = −0.403, p < .001); similarly, age of B2 was inversely correlated with BMI (Spearman R = −0.370, p < .001). When examining the regression model for age at menarche, BMI accounted for 11% of the variance, whereas race/ethnicity accounted for 6%.
Overall, the median tempo (age at B2 to menarche) was 2.7 years with an interquartile range (IQR) of 2.0-3.5 years (Table 3, data in months). Tempo was slower (interval of age of breast development to menarche was longer) in those with earlier onset of breast development; for example, the median tempo was 1.4 years longer in those with breast development prior to 8.5 years of age contrasted to those with breast development after 10.5 years (p < .001) (Table 3). Examination of the relationship between girls’ characteristics and tempo identified significant differences according to race/ethnicity and study site. The median tempos for Black and White girls were similar (median 2.9 and 3.0 years, respectively). However median tempo was shorter for Hispanic and Asian girls (Table 3). The median tempo among Cincinnati girls was 3.4 years, while New York and California girls had similar shorter tempos of 2.4 and 2.6 years, respectively. Although attenuated, the difference in Ohio girls persisted after adjustment and stratification by timing of B2 (data not shown).
Table 3:
Unadjusted and Mutually Adjusted estimates for tempo (months) associated with Race, Site, BMI, B2-age and Menarche Age
| Unadjusted | Mutually Adjusted | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | Median tempo (IQR) | Beta* (95%) | p value | Beta* (95%) | p value | |
| All | 906 | 33.0 (24.5, 42.5) | |||||
| Race/Ethnicity | Black | 278 | 35.0 (26.0,46.0) | ref | ref | ||
| Hispanic | 269 | 27.0 (19.5,37.0) | −7.64 (−9.89,−5.38) | <.0001 | −5.64 (−7.97,−3.31) | <.0001 | |
| Asian | 53 | 31.0 (23.5,36.5) | −5.40 (−9.35,−1.46) | 0.007 | −2.58 (−6.66,1.49) | 0.214 | |
| White | 306 | 36.0 (28.0,45.0) | 0.52 (−1.66,2.70) | 0.642 | 0.03 (−2.28,2.34) | 0.979 | |
| Site | NY | 276 | 29.0 (20.5,39.5) | ref | ref | ||
| OH | 260 | 40.5 (30.5,49.3) | 9.85 (7.60,12.10) | <.0001 | 6.91 (4.21,9.61) | <.0001 | |
| CA | 370 | 31.0 (24.0,39.5) | 1.18 (−0.89,3.25) | 0.264 | −0.33 (−2.72,2.06) | 0.785 | |
| Base-line BMI% | <50th | 298 | 33.0 (25.5,42.5) | ref | ref | ||
| 50-84.9th | 312 | 32.0 (24.3,42.0) | −0.53 (−2.74,1.68) | 0.637 | −0.19 (−2.28,1.89) | 0.856 | |
| 85-94.9th | 141 | 34.0 (25.0,44.5) | 1.24 (−1.55,4.02) | 0.384 | 1.62 (−1.02,4.26) | 0.228 | |
| >95th | 154 | 31.8 (23.0,47.0) | 1.58 (−1.12,4.29) | 0.251 | 3.11 (0.49,5.72) | 0.020 | |
| B2 Age | <8.5 yr | 253 | 42.5 (33.0,53.0) | ref | |||
| 8.5-10.5 yr | 487 | 31.0 (24.0,41.0) | −10.7 (−12.6,−8.84) | <.0001 | |||
| 10.5+ yr | 166 | 25.3 (17.0,32.0) | −17.6 (−20.1,−15.2) | <.0001 | |||
| Men-arche Age | <12 yr | 389 | 28.0 (20.0,36.0) | ref | |||
| 12 - 14 yr | 448 | 36.8 (28.3,45.5) | 9.07 (7.32,10.83) | <.0001 | |||
| 14+ yr | 69 | 42.0 (32.0,52.5) | 15.63 (12.33,18.94) | <.0001 | |||
Beta can be interpreted as change in months, computed using PROC GLM. Negative beta denotes shorter interval as well as faster tempo.
Adjusted models did not include B2-age or menarche age.
Discussion
Age at menarche can be determined by several approaches, including status quo, retrospective, and prospective methods,7 and it can be elicited from a parent or an adolescent, or recalled by an adult. Several studies have examined accuracy in recalled age at menarche, with correlations of .60-.83 (reviewed by Dorn)8; accuracy is typically greater with recall over shorter intervals after menarche.9 Data obtained through in-person interviews appear to be more reliable than those obtained through telephone interview.10 In this unique prospective study, we had the opportunity to collect information in person on age at menarche through reports by both the parent and in most cases by the girl herself over the course of several years for 1088 girls. We found that the data derived through multiple reports from both parent/guardian and the participant, were very consistent and differed little (0.18 years between parent/guardian and participant), likely well within the precision of any method to determine age at menarche. Most of our menarche ages were obtained from parents whose report was collected closer to the onset of menses, as girls were asked only later in the study; as noted, reports from girls were reliable nonetheless.
Menarcheal ages observed in our study are similar to those reported in the U.S. since 1990 (Table 4). Our median ages at menarche for blacks (12.0 years) and whites (12.7 years) are similar to those in the Pediatric Research in Office Settings (PROS) study in 1993 (12.2 years in blacks and 12.8 whites).11 During the last halfcentury, there has been a modest decrease in age at menarche over time, substantially less than the decrease noted in age at thelarche. For example, a group of experts observed that there was a younger age at menarche by 2.5-4 months over the past 25 years.12 Two subsequent reports on longitudinal studies of US youth noted similar decreases in age at menarche. Examining iterations of NHES/NHANES series, Krieger reported a significant increase in the proportion of adolescents with menarche under 11 years age over the 50 years between 1959 and 2008 (white adolescents, 2.6% to 6.7%; black adolescents, 4.6% to 12.2%).13 Similarly, Finer reported on the National Survey of Family Growth from 1939 to 1993; the mean age at menarche was 12.5 years in 1939, 12.6 years in the 1963 cohort, and fell to 12.3 years in 1993.14
Table 4.
Historical ages breast development (B2), and menarche, and imputed tempo, 1948-2017
| Author | Age of B2 |
Menarche | Interval B2 to menarche |
Correlation (R), B2- menarche |
N/location/year of birth | Designa |
|---|---|---|---|---|---|---|
| Reynolds & Wines, 194820 |
10.8 | 12.9 | 2.1 | 0.86 | 49 US |
L |
| Marshall & Tanner, 196921 |
11.2 | 13.5 | 2.3 | 0.65 | 192 UK |
L |
| Hägg et al, 199122 | 11.0 | 13.1 | 2.1 | 90 Sweden |
L | |
| Largo & Prader, 198323 | 10.9 | 13.4 | 2.2 | 0.47 | 142 Switzerland 1954-1980 |
L |
| Herman-Giddens et al, 199711 |
10.0 w 8.9 b |
12.9 12.2 |
2.9 3.3 |
17077 US 1980-1989 PROS |
C | |
| Marti-Henneberg & Vizmasnos, 199724 |
10.6 | 12.6 | 2.0 | 163 Spain, 1987 |
C | |
| Biro et al, 200619 | 10.2 w 9.6 b |
12.6 12.0 |
2.4 2.4 |
0.38 | 615w, 541b US 1986-1987, 10 yr |
L |
| Aksglaede et al, 200916 | 9.9 | 13.1 | 3.4 | 995 Denmark 1986-2002 |
C | |
| Christensen et al, 201025 |
10.2 | 12.9 | 2.7 | 3938 UK 1991-1992 ALSPAC |
L | |
| Current study: BCERP |
9.7 w 8.8 b |
12.5 w 11.8 b |
3.0 w 2.9 b |
0.55 | 1089 US 1996-2001 |
L |
L=longitudinal study, C=cross-sectional study.
Many studies, including ours,15 as well as international studies (such as the Copenhagen Puberty Study),16 have noted recently a much more robust decrease in age of breast maturation, even beyond the PROS study.11 Younger age at B2, with a smaller decrease in age at menarche, would be expected to lead to longer duration and slower “tempo” and, potentially, to extend the proposed pubertal window of susceptibility for adult morbidities, specifically breast cancer.17 A complementary explanation for slower tempo is that breast development could result from estrogen or estrogen-like effects on the body, as well as breast tissue, without earlier emergence of the GnRH (gonadotropin releasing hormone) pulse generator16; this explanation is consistent with the observed lack of increase in gonadotropin levels comparing an earlier to the later cohort of Copenhagen girls16 and the relatively lower estrogen concentrations in overweight and obese girls in early puberty,18 suggesting extragonadal estrogen production or exposures to xenoestrogens, or other factors such as dietary intake and physical activity.
Age at onset of breast maturation and age at menarche are two related milestones of puberty, but are not interchangeable. There is a significant association of these events, but the statistical correlation may have decreased over the past several decades.19 Of interest, this study noted a correlation closer to the older studies (i.e. 0.547 vs ~0.6 in ref. 19) than recent reports (r~0.4). Additionally, the interval between the events (i.e., tempo of puberty) may have increased in recent decades. Previous studies have reported later ages at onset of breast development compared to more contemporary studies; these earlier studies have noted a shorter tempo from breast onset to menarche (Table 4). In a similar fashion, contemporary longitudinal studies from Spain,24 Greece,26 England,25 and the United States,19 similar to our study, have noted the interval is longer in girls with an earlier age at breast development.
There are several potential limitations to our study. This study is not nationally representative, but does include broad racial and ethnic, as well as socioeconomic, diversity. The study sites differed from each other by racial and ethnic representation, by BMI, and by study visit interval (Cincinnati site was every 6, rather than 12, months during the first 5 years of exams). Although there was an apparent effect by site on age at menarche, the differences were attenuated after adjusting for other factors; thus this association is likely explained by differences in race and ethnicity, and BMI. Future studies can explore the influence of environmental exposures on both ages of breast development6 and menarche27 and thus the tempo through puberty.
Our study provides contemporary information on age at menarche in US girls, demonstrating that it has decreased minimally over the last 20 years. We noted that parental and adolescent reports of menarche, using our algorithm, were highly correlated, and median ages contrasting reports of parents and adolescents were within 0.2 years. Similar to our previous paper examining age of breast maturation, we found that BMI had a greater effect on age at menarche than did race and ethnicity, providing more evidence to support efforts to address the obesity epidemic among children in the US. Lastly, girls with earlier breast maturation experienced a slower tempo through puberty, which potentially could impact adult morbidity through an expanded window of susceptibility.
Acknowledgments
Funding sources: Funded by the National Institutes of Health (NIH), grants U01ES019435, U01ES019453, U01 ES019454, UL1RR024131, UL1RR026314, UL1RR029887.
Abbreviations:
- BMI
body mass index
- NY
New York
- OH
Ohio
- CA
California
- B2
age at breast stage 2
- US
United States
- IQR
interquartile range
Footnotes
Contributor’s statements:
Dr Biro, Dr Wolff, and Dr Teitelbaum conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript.
Ms Pajak collected data, carried out the initial analyses, and reviewed and revised the manuscript.
Dr Pinney, Dr Windham, Dr Galvez, Dr Greenspan, and Dr Kushi conceptualized and designed the study, and reviewed and revised the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Frank M Biro, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati OH 45229.
Ashley Pajak, Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY 10029.
Mary S Wolff, Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY 10029.
Susan M Pinney, Department of Environmental Health, University of Cincinnati College of Medicine, Cincinnati, OH 45267-0056.
Gayle C Windham, Division of Environmental and Occupational Disease Control, California Department of Public Health, Richmond, CA 94804.
Maida P Galvez, Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY 10029.
Louise C Greenspan, Department of Pediatrics, Kaiser Permanente, San Francisco, CA.
Larry H Kushi, Division of Research, Kaiser Permanente, Oakland, CA 94612.
Susan L Teitelbaum, Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY 10029.
BIBLIOGRPHY:
- 1.Graber JA. Pubertal timing and the development of psychopathology in adolescence and beyond. Horm Behav. 2013;64(2):262. [DOI] [PubMed] [Google Scholar]
- 2.Lee YN, Styne D. Influences on the onset and tempo of puberty in human beings and implications for adolescent psychological development. Horm Behav. 2013;64(2):250. [DOI] [PubMed] [Google Scholar]
- 3.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev.1993; 15(1):36. [DOI] [PubMed] [Google Scholar]
- 4.Must A, Phillips SM, Naumova EN, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am JEpidemiol. 2002;155(7):672. [DOI] [PubMed] [Google Scholar]
- 5.Biro FM, Galvez MP, Greenspan LC, et al. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. September 2010;126(3):e583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff MS, Teitelbaum SL, Pinney SM, et al. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environ Health Perspect. 2010;118(7):1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hediger ML, Stine RA. Age at menarche based on recall information. Ann Hum Biol. 1987;14(2):133. [DOI] [PubMed] [Google Scholar]
- 8.Dorn LD, Dahl RE, Biro F. Defining the boundaries of early adolescence: a user’s guide to assessing pubertal status and pubertal timing in research with adolescents. ApplDevelSci. 2006;10(1):30. [Google Scholar]
- 9.Koo MM, Rohan TE. Accuracy of short-term recall of age at menarche. Ann Hum Biol. 1997;24(1):61. [DOI] [PubMed] [Google Scholar]
- 10.Dorn LD, Sontag-Padilla LM, Pabst S, et al. Longitudinal reliability of self-reported age at menarche in adolescent girls: variability across time and setting. Dev Psychol. 2013;49(6):1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99(4):505. [DOI] [PubMed] [Google Scholar]
- 12.Euling SY, Herman-Giddens ME, Lee PA, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(Suppl 3):S172. [DOI] [PubMed] [Google Scholar]
- 13.Krieger N, Kiang MV, Kosheleva A, et al. Age at menarche: 50-year socioeconomic trends among US- born black and white women. Am J Public Health. 2015;105(2):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finer LB, Philbin JM. Trends in ages at key reproductive transitions in the United States, 1951–2010. Womens Health Issues. 2014;24(3):e271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biro FM, Greenspan LC, Galvez MP, et al. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132(6):1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aksglaede L, Sorensen K, Petersen JH, et al. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics. 2009;123(5):e932. [DOI] [PubMed] [Google Scholar]
- 17.Berkey CS, Frazier AL, Gardner JD, et al. Adolescence and breast carcinoma risk. Cancer. 1999;85(11):2400. [DOI] [PubMed] [Google Scholar]
- 18.Biro FM, Pinney SM, Huang B, et al. Hormone changes in peripubertal girls. J Clin EndocrinolMetab. July 16 2014;99(10):3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biro FM, Huang B, Crawford PB, et al. Pubertal correlates in black and white girls. J Pediatr. 2006;148(2):234. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds EL, Wines JV. Individual differences in physical changes associated with adolescence in girls. Am JDis Child. 1948;75(3):329. [DOI] [PubMed] [Google Scholar]
- 21.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hägg U, Karlberg J, Taranger J. The timing of secondary sex characters and their relationship to the pubertal maximum of linear growth in girls. Swed Dent J. 1991; 15(6):271. [PubMed] [Google Scholar]
- 23.Largo RH, Prader A. Pubertal development in Swiss girls. Helv Paediatr Acta. 1983;38(3):229. [PubMed] [Google Scholar]
- 24.Marti-Henneberg C, Vizmanos B. The duration of puberty in girls is related to the timing of its onset. J Pediatr. 1997;131(4):618. [DOI] [PubMed] [Google Scholar]
- 25.Christensen KY, Maisonet M, Rubin C, et al. Progression through puberty in girls enrolled in a contemporary British cohort. JAdolesc Health. 2010;47(3):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantsiotou S, Papadimitriou A, Douros K, et al. Maturational tempo differences in relation to the timing of the onset of puberty in girls. Acta Paediatr. 2008;97(2):217. [DOI] [PubMed] [Google Scholar]
- 27.Wolff MS, Pajak A, Pinney SM, et al. Associations of urinary phthalate and phenol biomarkers with menarch in a multiethnic cohort of young girls. Reprod Toxicol. 2016;67:56. [DOI] [PMC free article] [PubMed] [Google Scholar]