Abstract
Purpose
Patients with metastatic rhabdomyosarcoma (RMS), except those younger than 10 years with embryonal RMS, have an estimated long-term event-free survival (EFS) of less than 20%. The main goal of this study was to improve outcome of patients with metastatic RMS by dose intensification with interval compression, use of the most active agents determined in phase II window studies, and use of irinotecan as a radiation sensitizer.
Patients and Methods
Patients with metastatic RMS received 54 weeks of therapy: blocks of therapy with vincristine/irinotecan (weeks 1 to 6, 20 to 25, and 47 to 52), interval compression with vincristine/doxorubicin/cyclophosphamide alternating with etoposide/ifosfamide (weeks 7 to 19 and 26 to 34), and vincristine/dactinomycin/cyclophosphamide (weeks 38 to 46). Radiation therapy occurred at weeks 20 to 25 (primary) but was also permitted at weeks 1 to 6 (for intracranial or paraspinal extension) and weeks 47 to 52 (for extensive metastatic sites).
Results
One hundred nine eligible patients were enrolled, with a median follow-up of surviving patients of 3.8 years (3-year EFS for all patients, 38% [95% CI, 29% to 48%]; survival, 56% [95% CI, 46% to 66%]). Patients with one or no Oberlin risk factor (age > 10 years or < 1 year, unfavorable primary site of disease, ≥ three metastatic sites, and bone or bone marrow involvement) had a 3-year EFS of 69% (95% CI, 52% to 82%); high-risk patients with two or more risk factors had a 3-year EFS of 20% (95% CI, 11% to 30%). Toxicity was similar to that on prior RMS studies.
Conclusion
Patients with metastatic RMS with one or no Oberlin risk factor had an improved 3-year EFS of 69% on ARST0431 compared with an historical cohort from pooled European and US studies; those with two or more risk factors have a dismal prognosis, and new approaches are needed for this very-high-risk group.
INTRODUCTION
Although improvements in treatment strategies for patients with localized rhabdomyosarcoma (RMS) have occurred over the past 3 decades by decreasing toxicity without compromising cure, little progress has been made in curing patients with metastatic disease.1-5 Patients with stage 4 disease, with the exception of those younger than 10 years who have embryonal RMS (ERMS), have long-term event-free survival (EFS) of less than 20%.6-8 In a pooled analysis of European and North American studies, EFS ranged from less than 5% to 50% depending on the presence of risk factors, including age, primary site of disease, number of metastatic sites, and bone or bone marrow involvement.7 Neither intensification of chemotherapy by increasing the dose of cyclophosphamide and adding active agents to standard vincristine/actinomycin/cyclophosphamide (VAC) therapy nor use of high-dose chemotherapy with stem-cell rescue has improved outcome over the past 30 years.9
The Intergroup Rhabdomyosarcoma Study Group (IRSG) and its successor, the Children's Oncology Group (COG) Soft Tissue Sarcoma Committee, used a series of phase II up-front window studies to investigate new agents in patients with metastatic RMS, in which new agent combinations were followed by standard (VAC) therapy. This approach successfully identified ifosfamide/etoposide (IE), ifosfamide/doxorubicin (ID), vincristine/melphalan, topotecan, and topotecan/cyclophosphamide as active combinations.10 However, this window strategy did not improve the overall outcome of this high-risk group of patients. The last window trial, D9802, with vincristine/irinotecan (VI) as the window, had a complete or partial response rate of 70%, the highest of any combination tested to date, which merited additional investigation.11 This combination also was active in patients with recurrent disease.12 Moreover, irinotecan is a radiation-sensitizing agent, with concurrent radiation and irinotecan used in a variety of other malignancies.13-16 Vincristine/doxorubicin/cyclophosphamide (VDC) alternating with IE is considered effective therapy for patients with intermediate-risk RMS, and intensification of therapy by interval compression (cycles beginning every 14 days) that used this combination of drugs was safe and improved the outcome of patients with localized Ewing sarcoma compared with standard dosing every 21 days.17,18
The study described here, COG ARST0431, combined three strategies to improve the outcome of patients with metastatic RMS: dose intensification by interval compression, use of the most active agents determined in phase II window studies, and use of irinotecan as a radiation sensitizer. The primary objective of COG ARST0431 was to improve the early disease control interval for patients with metastatic RMS by using an intensive interval compression therapy to permit maximal early intensive exposure to multiple known active agents. Additional objectives of this study were to determine the feasibility of this approach by assessing immediate and short-term adverse effects of concomitant irinotecan and radiation delivery in previously untreated metastatic RMS.
PATIENTS AND METHODS
Eligibility
All patients younger than 50 years with newly diagnosed metastatic RMS who had received no prior chemotherapy or radiation therapy were eligible. Adequate cardiac, renal, and liver functions were required. Prior enrollment on COG biology study D9902 was required to confirm histologic eligibility for the treatment study. Patients with uncontrolled infections and women who were pregnant or actively breastfeeding were ineligible. The trial was approved by the Pediatric Central Review Board and by the institutional review boards of each participating institution, as required. Informed consent from the patient or parent/guardian assent as appropriate was obtained before enrollment.
Chemotherapy
The 54-week chemotherapy treatment schema and doses are shown in Table 1 along with time of reporting periods. Patients began therapy with two cycles of VI followed by interval-compressed treatment with VDC and IE up to a cumulative dose of 375 mg/m2 of anthracycline and 45 g/m2 of ifosfamide, after which treatment was completed with four cycles of VAC and two more cycles of VI. Toxicity data were collected during four reporting periods (RPs) on the basis of the Common Terminology Criteria for Adverse Events (version 3). RP1 included toxicity data for weeks 1 to 6 (VI), when only patients with intracranial extension and evidence of cranial nerve palsies or paraspinal lesions with cord compression received radiation. RP2 covered weeks 7 to 19, when patients received interval-compressed VDC/IE therapy and no VI. RP3 spanned weeks 20 to 34, with radiation given from week 20 to 26 with concurrent VI followed by continued interval-compressed VDC/IE therapy. During RP4 (weeks 35 to 54), patients received VAC chemotherapy followed by two final cycles of VI. Early in the study, irinotecan 20 mg/m2 was given (max dose, 40 mg/d) for 5 days for 2 consecutive weeks [(d × 5) × 2]. When the results of COG ARST0121 demonstrated that the more convenient schedule of 50 mg/m2 daily for 5 days (d × 5) was equivalent to the more protracted (d × 5) × 2 schedule, the study was amended to use the shorter d × 5 schedule.12
Table 1.
Treatment Schema and Chemotherapy Doses
| Week | Treatment |
|---|---|
| RP1 | |
| 1* | V, irin |
| 2 | V |
| 3 | V |
| 4 | V, irin |
| 5 | V |
| 6 | EVAL |
| RP2 | |
| 7 | VDC |
| 8 | V |
| 9 | IE |
| 10 | |
| 11 | VDC |
| 12 | V |
| 13 | IE |
| 14 | |
| 15 | VDC |
| 16 | V |
| 17 | IE |
| 18 | |
| 19 | EVAL |
| RP3 | |
| 20 | RT, V, irin |
| 21 | RT, V |
| 22 | RT, V |
| 23 | RT, V, irin |
| 24 | RT, V |
| 25 | RT |
| 26 | IE |
| 27 | |
| 28 | VDC |
| 29 | V |
| 30 | IE |
| 31 | |
| 32 | VDC |
| 33 | V |
| 34 | EVAL |
| RP4 | |
| 35 | VAC |
| 36 | |
| 37 | |
| 38 | VAC |
| 39 | |
| 40 | |
| 41 | VAC |
| 42 | V |
| 43 | V |
| 44 | VAC |
| 45 | |
| 46 | |
| 47† | V, irin |
| 48 | V |
| 49 | |
| 50 | V, irin |
| 51 | V |
| 52 | |
| 53 | |
| 54 | EVAL |
NOTE. Mesna will be used with cyclophosphamide and ifosfamide. Filgrastim will be used in VAC, VDC, and IE cycles. Sargramostim or peg-filgrastim should not be used. If there is an age change during treatment use the new appropriate age dosing in the next cycle.
Abbreviations: A, dactinomycin; C, cyclophosphamide; D, doxorubicin; E, etoposide; I, ifosfamide; irin, irinotecan; IV, intravenously; L, XXXX; RP, reporting period; RT, radiation therapy; V, vincristine.
Patients with evidence of intracranial extension should receive RT starting at week 1.
Previously unirradiated metastatic sites may be irradiated during weeks 47-51.
If tolerated (ie, no delay in administration of the next cycle because of delayed count recovery or delayed resolution of other toxicities and no serious toxicities), consider increasing to 75% and then to 100% of the calculated full dose.
At week 19 of therapy, patients were to receive radiation therapy to the primary site of disease for local control. Patients with intracranial extension and evidence of cranial nerve palsies or paraspinal lesions with cord compression could receive radiation therapy beginning at week 1. Metastatic sites of disease received radiation at week 19, or radiation was delayed until week 47. Primary as well as metastatic sites of disease were to receive 50.4 Gy of radiation. Although resection of the primary site was usually not feasible, surgery was recommended if it would minimize the radiation field or for symptom control.
Statistical Methods
The primary goal of this study was to improve outcomes for patients with metastatic RMS with intensive interval-compressed therapy with the use of known effective agents. EFS was defined as the time from study entry to the first occurrence of progression, relapse after response, or death as a result of any cause as a first event. Overall survival (OS) was defined as the time from study entry to death as a result of any cause. The Kaplan-Meier method was used to determine EFS and OS.19
For similar patients with high-risk RMS treated on the Intergroup Rhabdomyosarcoma Study-IV (IRS-IV) pilot with an ID-based regimen or with IRS-IV with IE-based regimens, the 3-year EFS was estimated to be 37%. These patients comprised an historical cohort for comparison with ARST0431. To determine whether particular subsets of patients benefited from the ARST0431 therapy regimen, we also analyzed outcome by number of risk factors according to Oberlin risk factor categories, which were age (< 1 or ≥ 10 years at diagnosis), unfavorable site (limbs or other), bone or bone marrow involvement, and three or more metastatic sites.7
Enrollment of 75 eligible patients was determined to provide approximately 90% power (testing at the 5% level of statistical significance [one-sided]) to detect an increase in EFS associated with a reduction in the failure risk to 60% of that with ID/IE therapy (corresponding to a 3-year EFS of 55%). ARST0431 included both efficacy and futility monitoring of EFS and compared the interim ARST0431 results with those of IRS-IV regimens with ID/IE. ARST0431 also specified toxicity monitoring of VI with radiation and the monitoring of the length of time it took for patients to proceed through protocol treatment. The study was monitored by a COG data and safety monitoring committee that recommended that the ARST0431 study continue as planned until it was released back to COG.
RESULTS
ARST0431 opened for patient enrollment on July 17, 2006, and completed accrual on June 13, 2008. One hundred nine eligible patients were enrolled: 20 received irinotecan on the (d × 5) × 2 schedule and 89, on the d × 5 schedule. Patient characteristics are summarized in Table 2. The majority of patients were older than 10 years (56% were age 10 to 20 years; 6% were age ≥ 21 years), were male (55%), had tumors that were mostly larger than 5 cm (80%) and showed alveolar histology (59%).
Table 2.
Patient and Tumor Characteristics
| Characteristic | No. (%) |
|---|---|
| Age, years | |
| < 1 | 3 (3) |
| 1-9 | 38 (35) |
| 10-20 | 61 (56) |
| ≥ 21 | 7 (6) |
| Sex | |
| Male | 60 (55) |
| Female | 49 (45) |
| Histology | |
| Alveolar | 64 (59) |
| Embryonal | 36 (33) |
| Other | 9 (8) |
| Primary site | |
| Extremity | 19 (17) |
| GI | 7 (6) |
| GU | 21 (19) |
| Head and neck | 4 (4) |
| Intrathoracic | 3 (3) |
| PM | 11 (10) |
| Perineum | 11 (10) |
| Retroperineum | 14 (13) |
| Trunk | 11 (10) |
| Other | 8 (7) |
| Tumor size, cm | |
| ≤ 5 | 22 (20) |
| > 5 | 87 (80) |
| Regional lymph node involvement | |
| No | 35 (32) |
| Yes | 65 (60) |
| Not evaluated | 9 (8) |
Abbreviations: GU, genitourinary; PM, parameningeal.
EFS and OS
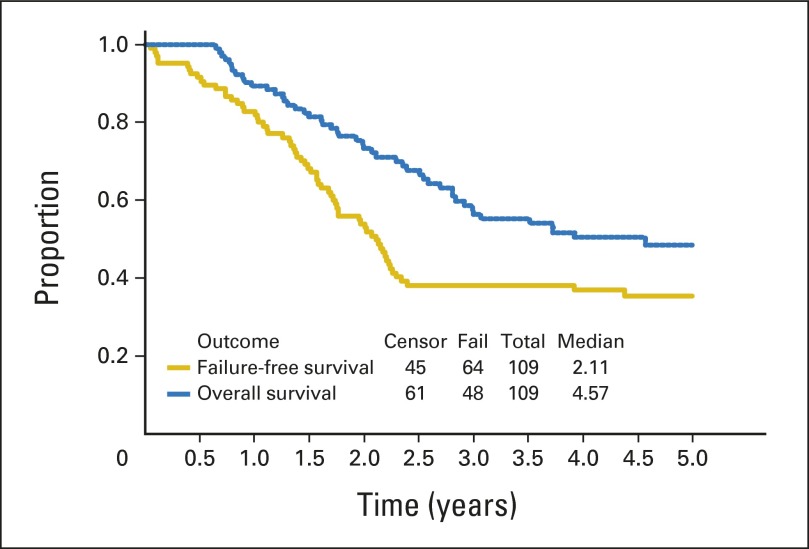
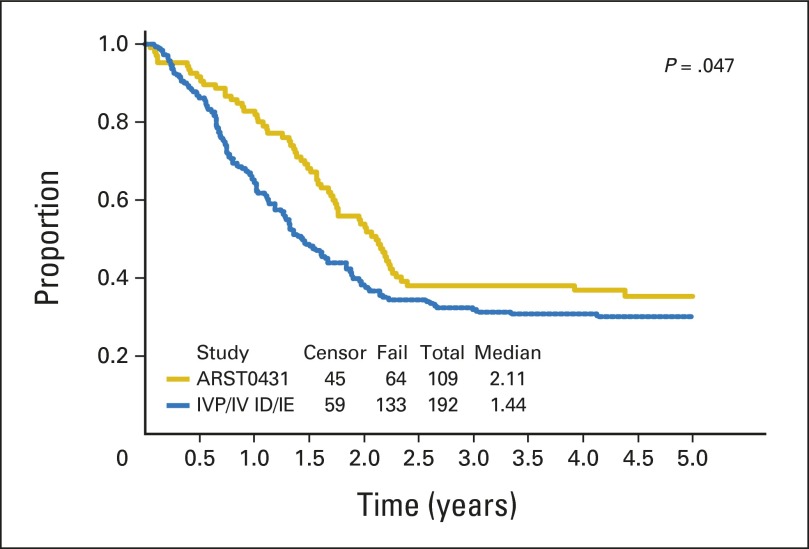
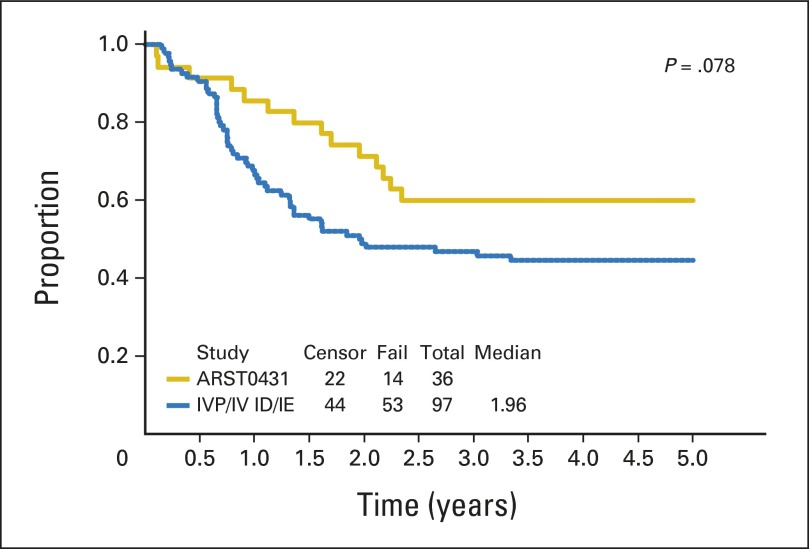
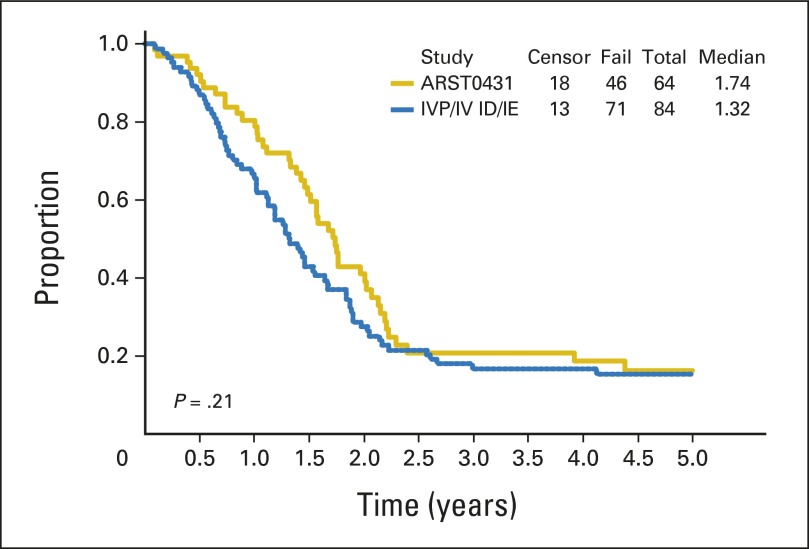
With a median follow-up of 3.8 years (range, 7.7 months to 4.7 years) in surviving patients, the 3-year EFS and OS for all patients were 38% (95% CI, 29% to 48%) and 56% (95% CI, 46% to 66%), respectively (Fig 1). Patient subgroup outcomes by Oberlin risk factor categories are shown in Table 3. Patients with one or no risk factors had the best outcome, with a 3-year EFS of 69% (95% CI, 52% to 82%), but patients with two or more risk factors had very poor outcome, with a 3-year EFS of only 20% (95% CI, 11% to 30%). The 20 patients with ERMS for fewer than 10 years at diagnosis had a 3-year EFS of 60% and a 3-year OS of 79%. With this subgroup excluded, the remaining patients on ARST0431 had a 3-year EFS of 32% (95% CI, 22% to 43%) and 3-year OS of 50% (95% CI, 38% to 61%). Outcome for patients who received irinotecan on the protracted (d × 5) × 2 schedule was similar to that for patients who received the shorter d × 5 schedule (data not shown); therefore, all data presented include all treated patients. When the EFS of patients on ARST0431 was compared with that of patients on the IRS-IV pilot with ID and IRS-IV with IE, only patients with ERMS showed an improved 5-year EFS (60% [95% CI, 42% to 74%] v 45% [95% CI, 34% to 54%]). The 5-year EFS for patients with alveolar RMS (ARMS) on ARST0431 and IRS-IV/IRS-IV pilot was similarly poor (16% [95% CI, 8% to 28%] and 15% [95% CI, 9% to 24%], respectively. Appendix Figs A1, A2, and A3, online only, show the comparison of EFS between IRS-IV/IRS-IV pilot and ARST0431 for all patients, for patients with ERMS, and for patients with ARMS, respectively.
Fig 1.
Outcome for all patients in ARST0431.
Table 3.
Outcome by Oberlin Risk Group
| Outcome | % (95% CI) by Risk Group |
||
|---|---|---|---|
| ≤ 1 Risk Factor (n = 43) | ≥ 2 Risk Factors (n = 66) | ERMS at Age < 10 Years (n = 20) | |
| 3-year EFS | 69 (52 to 82) | 20 (11 to 30) | 60 (36 to 78) |
| 3-year OS | 79 (62 to 89) | 14 (11 to 18) | 79 (54 to 92) |
Abbreviations: EFS, event-free survival; ERMS, embryonal rhabdomyosarcoma; OS, overall survival.
Feasibility and Toxicity
The duration of each treatment period, with the anticipated treatment duration, is shown in Table 4 by RP. Fourteen patients received radiation during RP1; 15, during RP2; 71, during RP3; and nine, during RP4. Twenty-five patients never received radiation because of withdrawal from protocol therapy (n = 3) or progressive disease (n = 4) during RP1; withdrawal from protocol therapy (n = 7), progressive disease (n = 2), or loss to follow-up (n = 1) during RP2; and withdrawal from protocol therapy during RP3 without radiation (n = 1) or completion of all therapy without radiation (n = 7).
Table 4.
RP Duration
| RP | 25th Percentile | Median | 50th Percentile |
|---|---|---|---|
| Weeks 1-6 (42 days) | 42 | 43 | 46 |
| Weeks 7-19 (91 days) | 98 | 109 | 119 |
| Weeks 20-34 (105 days) | 120 | 135 | 155 |
| Weeks 35-54 (140 days) | 136 | 145 | 161 |
Abbreviation: RP, reporting period.
Appendix Table A1, online only, details grades 3 to 5 nonhematologic toxicity by RP for toxicities that occurred in at least 5% of patients. Two deaths occurred in the study during RP3, one as a result of infection and one as a result of concurrent cytomegalovirus infection and radiation pneumonitis. The most common toxicity was infection with and without neutropenia in 50% to 60% of patients during RPs that contained VDC/IE, but this toxicity was rare during RP1, for which the regimen only comprised VI. Diarrhea was seen in 13% to 20% of patients during irinotecan-containing RPs but in only 2% of patients during RPs that did not contain VI.
DISCUSSION
With the exception of patients younger than age 10 years who had metastatic ERMS, the outcome for stage 4 RMS has been dismal. Several decades of clinical trials have failed to improve the overall outcome to greater than 30%. Attempts to intensify therapy for high-risk nonmetastatic RMS by the addition of carboplatin, epirubicin, and etoposide to ifosfamide/vincristine/dactinomycin, as well as up-front dose intensification with the addition of doxorubicin to ifosfamide/vincristine/dactinomycin, have not shown improvement in outcome for high-risk nonmetastatic RMS.20,21
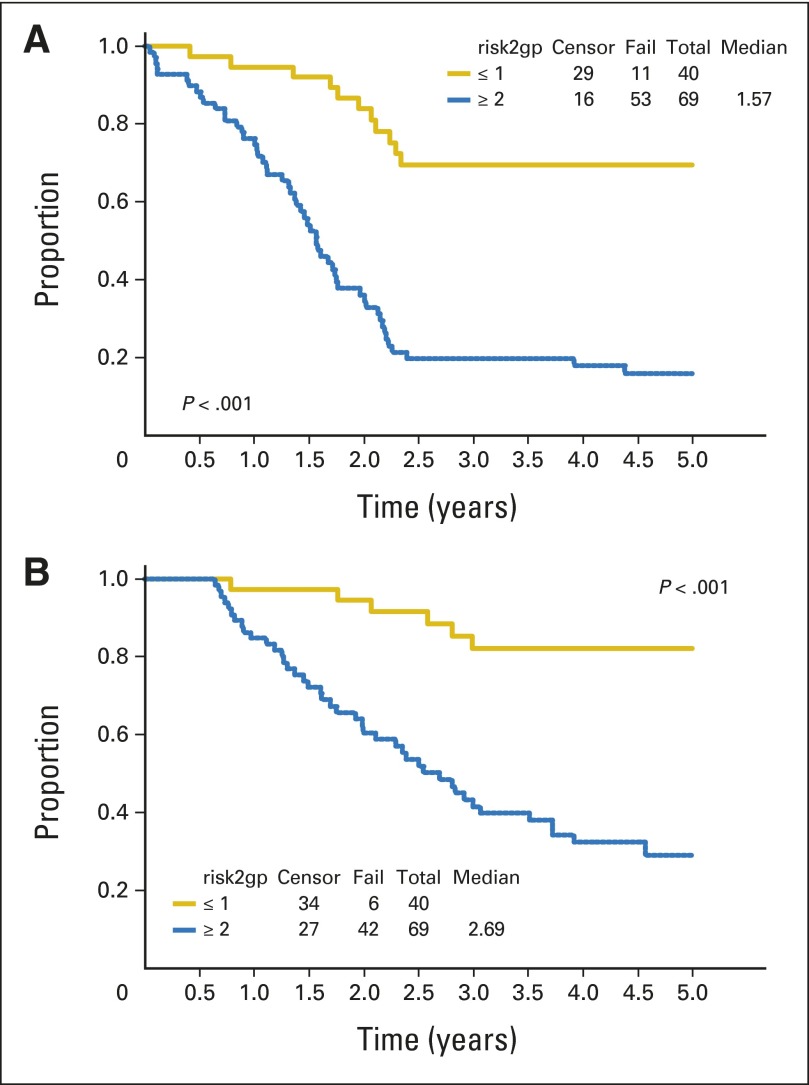
With use of a dose-intensive multiagent regimen, we report a 3-year EFS of 38% and OS of 56% for all patients with stage 4 RMS. In contrast, the most successful IRSG/COG phase II window studies that used ID/IE reported a 3-year EFS of 30% and 3-year OS of 43%. Compared with the historic IRSG/COG phase II window results, the apparent improvement in outcome in ARST0431 was in patients with ERMS only (Appendix Figs A1 to A3, online only). Patients with one or no Oberlin risk factors had an improved 3-year EFS of 67% (95% CI, 50% to 79%) on ARST0431 compared with 44% (95% CI, 38% to 49%) on the Oberlin cohort.7 Patients with two or more risk factors had no improvement in 3-year EFS on ARST0431 (19%; 95% CI, 10% to 30%) compared with the Oberlin cohort (14%; 95% CI, 11% to 18%).7 Use of historical comparisons has shortcomings when analyzing treatment regimens that resulted from changes in supportive care practices, changes in risk classifications, and lack of comparability of patient populations. In an attempt to control for these variables, we used the prognostic risk stratification devised by Oberlin et al7 from a large international data set to determine whether specific subgroups benefited from this approach. Patients younger than 10 years with ERMS had a 3-year EFS of 60%, which is similar to that seen on COG D9803, which used the less complicated VAC regimen (cyclophosphamide 2.2 g/m2/course).5 This is not surprising, because 17 of the 20 patients in this group had an Oberlin risk score of 0 to 1 (data not shown). When treated with ARST0431 therapy, patients younger than 10 years who had metastatic ERMS with an Oberlin risk score of 0 to 1 had an outcome similar to intermediate-risk patients without metastasis. One of the shortcomings of ARST0431 is the inability to determine which of the study interventions had the most effect: interval compression, use of the most active combinations from phase II windows, or use of irinotecan during radiation. Patients with two or more risk factors had a 3-year EFS of 19% on ARST0431 and of 14% in the Oberlin cohort; thus, they did not benefit from this approach. Figure 2 shows the outcomes for patients treated on ARST0431 by Oberlin risk group.
Fig 2.
Outcome by Oberlin risk group (risk2gp): (A) event-free survival; (B) overall survival.
Toxicity of the regimen was as expected. The most common nonhematologic grade 3 or higher toxicity was diarrhea, which occurred in up to 20% of irinotecan-containing RPs, and febrile neutropenia with or without documented infection, which occurred in 53% of patients during RP2 and 63% during RP3. The longest delays in chemotherapy administration occurred during weeks 20 to 34, when patients received radiation therapy to the primary tumor site.
We have identified an expanded group of patients with low-risk metastatic disease (Oberlin risk score 0 to 1) who have a 3-year EFS of 67% when treated with a dose-intensive multiagent regimen that included irinotecan, with irinotecan given during radiation. This expanded group includes patients with ERMS age 10 years and older but who nonetheless have an Oberlin score of less than 2. These results represent an apparent improvement over an historical cohort. Different approaches are needed for the remainder of the high-risk patients with ARMS who do not benefit from current treatment strategies. Unfortunately, ARMS has fewer genetic aberrations than ERMS and no known recurrently mutated cancer consensus genes, which limits genetic targets available for therapeutic approaches. Oxidative stress has been identified as a target of therapeutic relevance to be explored for high-risk ERMS.22,23 In addition, a majority of RMS tumors alter the receptor tyrosine kinase axis, which provides an opportunity for therapeutic intervention.24 These approaches will be explored in future studies in high-risk RMS.
Supplementary Material
GLOSSARY TERM
- rhabdomyosarcoma:
a malignant solid tumor arising from mesenchymal tissues, which typically differentiate to form striated muscle. Rhabdomyosarcoma is divided into two primary subtypes: the alveolar rhabdomyosarcoma, primarily arising in adolescents and young adults, and the embryonal rhabdomyosarcoma, predominantly affecting infants and children. It is one of the most frequently occurring soft-tissue sarcomas and the most common in children younger than age 15 years, accounting for 6% to 8% of all childhood cancers.
Appendix
Table A1.
Grades 3 to 5 Toxicities Seen in > 5% of Patients by RP
| Toxicity | No. (%) of Toxicities by RP* |
|||
|---|---|---|---|---|
| 1 (n = 107; weeks 1-6) | 2 (n = 98; weeks 7-19) | 3 (n = 87; weeks 20-34) | 4 (n = 70; weeks 35-54) | |
| Febrile neutropenia with documented infection | 3 (2.8) | 22 (22) | 27 (32) | 8 (11.4) |
| Febrile neutropenia, no documented infection | 2 (1.9) | 30 (30.6) | 27 (31.0) | 9 (12.9) |
| Diarrhea | 19 (17.8) | 2 (2.0) | 17 (19.5) | 9 (12.9) |
| Infection with grades 1-2 neutrophils, all sites | 14 (13) | 11 (11.2) | 13 (15) | 10 (14.2) |
| Anorexia | 14 (13.1) | 9 (9.2) | 10 (11.5) | 1 (1.4) |
| Dehydration | 11 (10.3) | 3 (3.1) | 5 (5.7) | 3 (4.3) |
| Hypokalemia | 9 (8.4) | 8 (8.2) | 10 (11.5) | 3 (4.3) |
| Mucositis oral examination | 3 (2.8) | 7 (7.1) | 5 (5.7) | |
| Nausea | 9 (8.4) | 6 (6.1) | 6 (6.9) | 4 (5.7) |
| Mucositis oral functional | 1 (0.9) | 6 (6.1) | 5 (6.9) | 1 (5.7) |
| Weight loss | 3 (2.8) | 2 (2.0) | 6 (6.9) | 4 (5.7) |
| Radiation dermatitis | 66.9 | |||
| Abdominal pain | 7 (6.5) | 2 (2) | 6 (6.9) | 2 (2.9) |
| Vomiting | 6 (5.6) | 4 (4.1) | 5 (5.7) | 5 (7.1) |
| Sensory neuropathy | 2 (2) | 5 (5.7) | 1 (1.4) | |
| ALT elevation | 5 (4.7) | 3 (3.1) | 5 (5.7) | 2 (2.9) |
| Colitis | 6 (5.6) | 1 (1.1) | 1 (1.4) | |
| Eligible patients with grades 3-5 toxicity in course | 57 (52) | 67 (68) | 69 (79) | 39 (55) |
Abbreviation: RP, reporting period.
Numbers of patients in each RP are the total number of patients with data for that RP.
Fig A1.
Event-free survival: ARST0431 versus Intergroup Rhabdomyosarcoma Study-IV (IRS-IV) pilot (IVP; ifosfamide/doxorubicin [ID])/IRS-IV (ifosfamide/etoposide [IE]).
Fig A2.
Event-free survival: ARST0431 versus Intergroup Rhabdomyosarcoma Study-IV (IRS-IV) pilot (IVP; ifosfamide/doxorubicin [ID])/IRS-IV (ifosfamide/etoposide [IE]; embryonal rhabdomyosarcoma only).
Fig A3.
Event-free survival: ARST0431 versus Intergroup Rhabdomyosarcoma Study-IV (IRS-IV) pilot (IVP; ifosfamide/doxorubicin [ID])/IRS-IV (ifosfamide/etoposide [IE]; alveolar rhabdomyosarcoma only).
Footnotes
See accompanying editorial on page 105
Supported by Children's Oncology Group Grants No. U10CA180886, U10CA180899, U10CA098543, and U10CA098413.
Terms in blue are defined in the glossary, found at the end of this article, and online at www.jco.org.
Presented in part at the meeting of the Connective Tissue Oncology Society, Prague, Czech Republic, November 14-17, 2012, and at the 46th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 4-8, 2010.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00354744.
AUTHOR CONTRIBUTIONS
Conception and design: Brenda J. Weigel, James R. Anderson, William H. Meyer, David A. Rodeberg, Douglas S. Hawkins, Carola A.S. Arndt
Collection and assembly of data: Brenda J. Weigel, James R. Anderson, David M. Parham, David A. Rodeberg, Jeff M. Michalski, Carola A.S. Arndt
Data analysis and interpretation: Brenda J. Weigel, Elizabeth Lyden, James R. Anderson, David A. Rodeberg, Douglas S. Hawkins, Carola A.S. Arndt
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Intensive Multiagent Therapy, Including Dose-Compressed Cycles of Ifosfamide/Etoposide and Vincristine/Doxorubicin/Cyclophosphamide, Irinotecan, and Radiation, in Patients With High-Risk Rhabdomyosarcoma: A Report From the Children's Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Brenda J. Weigel
Travel, Accommodations, Expenses: Genentech, Eli Lilly/ImClone Systems
Elizabeth Lyden
No relationship to disclose
James R. Anderson
Employment: Merck
Consulting or Advisory Role: SFD Pharmaceuticals, Merck, Amgen (I)
William H. Meyer
No relationship to disclose
David M. Parham
No relationship to disclose
David A. Rodeberg
No relationship to disclose
Jeff M. Michalski
Travel, Accommodations, Expenses: ViewRay, Siemens Healthcare Diagnostics, Varian Medical Systems, GE Health Care, Mevion Medical Systems
Douglas S. Hawkins
No relationship to disclose
Carola A.S. Arndt
Stock or Other Ownership: Merck, Pfizer
REFERENCES
- 1.Maurer HM, Beltangady M, Gehan EA, et al. The Intergroup Rhabdomyosarcoma Study-I: A final report. Cancer. 1988;61:209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Maurer HM, Gehan EA, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 4.Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 5.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's Oncology Group study D9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malempati S, Hawkins DS. Rhabdomyosarcoma: Review of the Children's Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer. 2012;59:5–10. doi: 10.1002/pbc.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: Results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26:2384–2389. doi: 10.1200/JCO.2007.14.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breneman JC, Lyden E, Pappo AS, et al. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol. 2003;21:78–84. doi: 10.1200/JCO.2003.06.129. [DOI] [PubMed] [Google Scholar]
- 9.Weigel BJ, Breitfeld PP, Hawkins D, et al. Role of high-dose chemotherapy with hematopoietic stem cell rescue in the treatment of metastatic or recurrent rhabdomyosarcoma. J Pediatr Hematol Oncol. 2001;23:272–276. doi: 10.1097/00043426-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Lager JJ, Lyden ER, Anderson JR, et al. Pooled analysis of phase II window studies in children with contemporary high-risk metastatic rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2006;24:3415–3422. doi: 10.1200/JCO.2005.01.9497. [DOI] [PubMed] [Google Scholar]
- 11.Pappo AS, Lyden E, Breitfeld P, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: The Children's Oncology Group. J Clin Oncol. 2007;25:362–369. doi: 10.1200/JCO.2006.07.1720. [DOI] [PubMed] [Google Scholar]
- 12.Mascarenhas L, Lyden ER, Breitfeld PP, et al. Randomized phase II window trial of two schedules of irinotecan with vincristine in patients with first relapse or progression of rhabdomyosarcoma: A report from the Children's Oncology Group. J Clin Oncol. 2010;28:4658–4663. doi: 10.1200/JCO.2010.29.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rich TA, Kirichenko AV. Camptothecin radiation sensitization: Mechanisms, schedules, and timing. Oncology. 1998;12:114–120. [PubMed] [Google Scholar]
- 14.Komaki R, Janjan NA, Ajani JA, et al. Phase I study of irinotecan and concurrent radiation therapy for upper GI tumors. Oncology. 2000;14:34–37. [PubMed] [Google Scholar]
- 15.Kirichenko AV, Rich TA. Radiation enhancement by 9-aminocamptothecin: The effect of fractionation and timing of administration. Int J Radiat Oncol Biol Phys. 1999;44:659–664. doi: 10.1016/s0360-3016(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 16.Choy H, Chakravarthy A, Devore RF, III, et al. Weekly irinotecan and concurrent radiation therapy for stage III unresectable NSCLC. Oncology. 2000;14:43–46. [PubMed] [Google Scholar]
- 17.Arndt CA, Nascimento AG, Schroeder G, et al. Treatment of intermediate risk rhabdomyosarcoma and undifferentiated sarcoma with alternating cycles of vincristine/doxorubicin/cyclophosphamide and etoposide/ifosfamide. Eur J Cancer. 1998;34:1224–1229. doi: 10.1016/s0959-8049(98)00080-x. [DOI] [PubMed] [Google Scholar]
- 18.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children's Oncology Group. J Clin Oncol. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Oberlin O, Rey A, Sanchez de Toledo J, et al. Randomized comparison of intensified six-drug versus standard three-drug chemotherapy for high-risk nonmetastatic rhabdomyosarcoma and other chemotherapy-sensitive childhood soft tissue sarcomas: Long-term results from the International Society of Pediatric Oncology MMT95 study. J Clin Oncol. 2012;30:2457–2465. doi: 10.1200/JCO.2011.40.3287. [DOI] [PubMed] [Google Scholar]
- 21.Bisogno C DSG, Bergeron C, Carli M, et al. The role of doxorubicin in the treatment of rhabdomyosarcoma: Preliminary results from the EpSSG RMS2005 randomized trial. Pediatr Blood Cancer. 2014;61:S133–S134. (suppl S2) [Google Scholar]
- 22.Hedrick E, Crose L, Linardic CM, et al. Histone deacetylase inhibitors inhibit rhabdomyosarcoma by reactive oxygen species-dependent targeting of specificity protein transcription factors. Mol Cancer Ther. 2015;14:2143–2153. doi: 10.1158/1535-7163.MCT-15-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Stewart E, Shelat AA, et al. Targeting oxidative stress in embryonal rhabdomyosarcoma. Cancer Cell. 2013;24:710–724. doi: 10.1016/j.ccr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shern JF, Chen L, Chmielecki J, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov. 2014;4:216–231. doi: 10.1158/2159-8290.CD-13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.