Abstract
This longitudinal study investigated whether greater pre-pubertal adiposity was associated with subsequent timing of maturation and bone strength during adolescence in 135 girls and 123 boys participating in the Iowa Bone Development Study. Greater adiposity was defined using BMI data at age 8 years to classify participants as overweight (OW, ≥85th percentile for age and sex) or healthy-weight, HW). Maturation was defined as the estimated age of peak height velocity (PHV) based on a series of cross-sectional estimates. Measurements were taken at ages 11, 13, 15 and 17 years for estimates of body composition by DXA, bone compression (bone strength index) and torsion strength (polar strength-strain index) at the radius and tibia by pQCT, and femoral neck bending strength (section modulus) by hip structural analysis. Bone strength in OW versus HW were evaluated by fitting sex-specific linear mixed models that included centered age (visit age – grand mean age of cohort) as the time variable and adjusted for change in fat mass, and limb length in Model 1. Analyses were repeated using biological age (visit age – age PHV) as the time variable for Model 1 with additional adjustment for lean mass in Model 2. BMI was negatively associated with age of maturation (p<0.05). OW versus HW girls had significantly greater bone strength (p<0.001) in Model 1, while OW versus HW boys had significantly greater bone strength (p<0.001) at the tibia and femoral neck, but not radius (p>0.05). Analyses were repeated using biological age, which yielded reduced parameter estimates for girls but similar results for boys (Model 1.) Differences were no longer present following adjustment for lean mass (Model 2) in girls (p>0.05) while differences at the tibia were sustained in boys (p<0.05). These findings demonstrate sex- and site-specific differences in the associations between adiposity, maturation and bone strength.
Keywords: Bone-fat interactions, Bone QCT, Osteoporosis, General population study, DXA
Introduction
The prevalence of obesity among children and adolescents has increased substantially over the last three decades.(1,2) While childhood obesity has been associated with greater risk for adverse cardiovascular and metabolic conditions,(3) it is unclear whether it is detrimental to bone strength during growth.
Several lines of evidence suggest greater adiposity may influence the timing of maturation,(4-9) and in doing so, could influence bone strength.(10-15) Obesity consistently has been associated with earlier maturation in girls(4-7) but both earlier and later maturation in boys.(4,6,8,9) Though a mechanism is still uncertain, greater adiposity could have an impact on hormone levels during growth, thereby influencing the timing of maturation. For example, greater adiposity could lead to higher circulating levels of estrogen due to increased aromatase conversion of androgens to estrogen in adipose tissue,(16,17) potentially increasing estrogen exposure during pre-pubertal years.(17) Insulin resistance also is associated with obesity, and high insulin levels stimulate ovarian production of estrogen and androgen and reduce liver production of sex hormone binding globulin (SHBG), which may result in higher levels of available estrogen.(18) In boys, however, it has also been suggested that increasing levels of estrogen could inhibit gonadotropin secretion,(19) thereby delaying maturation. Leptin, a factor secreted by adipocytes, could also play a role in early maturation with obesity since it may be a permissive factor for pubertal development(20,21) and is elevated in the setting of obesity.(20,22) Earlier puberty or physical maturation has been associated longitudinally with higher bone mass(10) and greater bone mineral accrual in girls,(11) and greater areal and volumetric bone mineral density in boys.(12) Delayed puberty or physical maturation, on the other hand, could have harmful effects on bone development in both girls(13,14) and boys.(15) Thus, obesity could lead to earlier maturation in girls, which is associated with greater bone strength, however the effect on maturation and bone development in boys remains unclear.
Childhood through adolescence is a critical period for bone development. The opportunity to optimize bone size, a potential determinant of bone strength in adulthood,(23,24) diminishes following completion of bone growth by the end of adolescence. Furthermore, an estimated 80 to 90% of peak bone mass is accrued by late adolescence(25) Consequently, suboptimal bone development during this time could impact peak bone mass, a significant determinant of risk for osteoporosis later in life.(26) Therefore, the objective of this study was to evaluate the longitudinal bone-fat relationship from age 11 to 17 years, and to primarily determine whether greater pre-pubertal adiposity was significantly associated with subsequent age of maturation and adolescent bone strength. We hypothesized that the bone-fat relationship would be positive in girls but negative in boys, and that greater, pre-pubertal adiposity would be associated with earlier subsequent age of maturation and greater bone strength in girls but a later age of maturation and lower bone strength in boys.
Subjects and Methods
The Iowa Bone Development Study (IBDS) is an observational, longitudinal study of the effects of fluoride and other factors on bone development from early childhood through young adulthood.(27) Children were enrolled at approximately 5 years of age from the Iowa Fluoride Study birth cohort,(28) and followed approximately every 2 to 3 years. Informed consent/assent was obtained from parents and their children using Institutional Review Board-approved forms. The present analyses were restricted to data collected from age 11 through 17 years when the study began to utilize pQCT, with the exception of the age 8 BMI data. Participants were excluded for a history of medication use or medical conditions that may affect bone metabolism or timing of maturation, as well as for not attending a baseline and age 15 or 17 follow-up visit.
Anthropometric Measurements
At each visit, height was measured in centimeters by stadiometer (Harpenden, Holtain, UK) and weight in kilograms using a balance beam scale (Healthometer physician's scale, Continental, Bridgeview, IL) by trained research nurses.
DXA Measurement of Body Composition and Femoral Neck Strength
Scans of the whole body and left hip were acquired using dual energy x-ray absorptiometry (DXA) at ages 11, 13, 15 and 17 on the Hologic QDR 4500 Delphi A (Hologic, Inc., Bedford, MA) using fan beam mode (software version 12.3). Participants were scanned wearing street clothes but without shoes or metal items by one of three trained technicians. Estimates of fat and lean mass (kg) were obtained from whole body DXA scans. Hip scans were reanalyzed using Hip Structural Analysis (HSA, APEX 4.0/13.4 Hologic software), which utilizes the 2-dimensional DXA data to estimate hip structure and strength. An estimate of femoral neck bending strength (section modulus, Z, cm3) was obtained from the DXA hip scan. This measurement was selected to provide an estimate of bone strength at an axial site. HSA estimates of section modulus have been found to correlate well with QCT estimates.(29) The Hologic spine phantom was scanned daily for quality assurance, and the whole body step phantom was scanned weekly per manufacturer guidelines to calibrate the machine for whole body composition.
pQCT Bone Measurements
Bone was evaluated using pQCT (XCT 2000 or 3000, Stratec Medizintechnik GmbH, Pforzheim, Germany, software version 6.2) at the radius (non-dominant side) and tibia beginning at age 11. A pQCT phantom was scanned daily for quality assurance and scans were performed by one of three trained technicians. Trained technicians measured the length of the forearm and tibia in millimeters (mm) with a standard ruler from the olecranon process to the ulnar styloid process for the radius and from the center of the medial malleolus to the end of the tibial plateau for the tibia. The pQCT scans were acquired using voxel size of 0.4 mm, scan speed of 20 mm/sec, and slice thickness of 2.4 mm. Scans were acquired at sites 4 and 20% of the limb length proximal from the distal radius (growth plate) and 4 and 38% of the limb length proximal from the distal end of the tibia. The reference line for the start of each scan was placed to bisect the medial border of the most proximal growth plate. When growth plates were no longer visible, the reference line was placed to bisect the medial border of the distal endplate. Trabecular bone mineral density (tBMD, mg/cm3) and an estimate of bone compressive strength, bone strength index (BSI, mg2/mm4),(30) were estimated from the 4% site (metaphysis). Bone at this site was measured using contour mode 3 with an attenuation threshold of 169 mg/cm3, to separate bone from soft tissue. Peel mode 4, which has an attenuation threshold of 650 mg/cm3 with a 10% peel was used to separate trabecular from total bone. Cortical bone mineral density (cBMD, mg/cm3), periosteal circumference (PC, mm) and cortical thickness (CT, mm) were estimated from the 20% radial and 38% tibial sites (diaphysis). In addition, an estimate of bone strength to resist torsion, polar strength-strain index (pSSI, mm3),(30,31) was estimated at the 20% radial and 38% tibial site. For these measurements, contour mode 1 and cortmode 2 with thresholds of 710 mg/cm3 and 480 mg/cm3 were applied. A circular ring model was used to obtain the cortical thickness measurement. Due to size constraints of the XCT 2000 for tibial scans, participants with a calf measurement greater than 15.5 inches were scanned at the tibia using the XCT 3000. A calibration study evaluating the 4 and 38% sites in 12 women and 5 men 21-58 years old was completed at our institution. Total bone area and density from the XCT 3000 was within 1.5% of XCT 2000 measurement and differed by less than 2.2% for SSI. To evaluate whether pQCT machine type had an influence on our findings, we repeated all longitudinal analyses for the tibia with an indicator variable for pQCT machine type. We found machine type was not significantly associated with any bone outcomes except for pSSI in comparisons of overweight (OW) versus healthy-weight (HW) girls and boys. In those cases, inclusion of this machine type variable slightly increased rather than decreased estimated differences between OW and HW girls and boys.
Scan quality was assessed by two trained technicians. This included review of proper reference line placement and progression of reference line placement between visits. Scans were also evaluated for motion and excluded if there was a visible break in the cortical rim with displacement of the rim.
Maturation Status
Physical maturity was defined as the age of peak height velocity (PHV), an objectively-measured maturational milestone that can be applied to both girls and boys and that has been previously utilized in longitudinal studies of bone outcomes during youth and adulthood.(11,12) Participants underwent standing and sitting height measurements. Leg length was estimated from these measurements at each assessment visit starting from age 11. The age of PHV was estimated from the age 11, 13 and 15 assessment visits using Mirwald's predictive equations.(32) This estimation method was selected since 98% of male and 96% of female IBDS participants included in analyses are white, and the Mirwald equation was validated in white Canadian children and adolescents. These equations include age, sex, weight, height, sitting height, and leg length as predictors. Age at PHV estimates were calculated for all participants using age 11 and 13 data for girls and age 13 and 15 data for boys, if available. The clinical examination (between age 11 and age 15) which provided an estimate of PHV age that was closest to the actual clinical examination age was used as the best estimate. If only one age of PHV estimate was available, it was used.
Physical Activity Level
The Physical Activity Questionnaire for Children (PAQ-C) and for Adolescents (PAQ-A) are seven-day recall questionnaires originally designed to measure self-reported physical activity levels in children 8-14 and 14-20 years old, respectively.(33,34) In this study, the PAQ-C was completed for age 11 measurement visits and the PAQ-A was completed at age 13 and older measurement visits. The PAQ-C and-A scores are means of all items on a scale of 1 to 5, with higher activity levels indicated by higher scores.
Adiposity Group Definition
BMI (kg/m2) was calculated using data obtained at the age 8 measurement visit and participants were defined as healthy weight (HW) or overweight/obese (OW) according to CDC percentiles for age and sex – healthy weight (5th< BMI percentile <85th), overweight/obese (overweight: ≥85th percentile <95th, obese: BMI percentile ≥95th).(35) The age 8 measurement visit BMI was selected to define pre-pubertal adiposity group since this visit preceded age of PHV in our cohort.
Statistical Methods
All analyses were stratified by sex. Characteristics of girls and boys were described for each measurement visit using mean±SD for continuous variables and percentage for categorical variables. The normality of variables was assessed using the Shapiro-Wilk test, histograms and Q-Q plots and no severe departures from normality were observed. Differences in continuous measures between girls and boys were evaluated using Student's t-tests, while distributions of categorical variables were compared using chi-square tests. SAS statistical software (version 9.3, SAS Institute, Inc., Cary, NC) was utilized for analyses and a p-value <0.05 was considered statistically significant.
Study Objective 1
To evaluate the longitudinal bone-fat relationship from age 11 to 17 years. The longitudinal relationship between fat mass and bone parameters from age 11 to 17 years was modeled by fitting linear mixed models without and with adjustment for covariates using the SAS MIXED procedure. In the mixed models, biological age, the number of years before or after achieving PHV ( measurement visit age – age of PHV), was used as the time variable. The intercept and slope for biological age were specified as random effects. The fixed effects included the time-varying predictor variable, fat mass, as a continuous variable, biological age as the time variable and limb length to account for differences among participants in size in one model (Model 1) plus arm lean mass for the forearm site and whole body lean mass for the tibial site and the femoral neck in a second model (Model 2). Additionally, biological age polynomial functions (i.e., biological age2 and biological age3) were included to allow for non-linearity of growth. Interaction effects between biological age and fat mass were evaluated. Physical activity level was considered for inclusion as a covariate in Model 2, but was not included due to lack of statistical significance. All variables included in the models, except for age, were standardized prior to model fitting (mean=0, SD=1).
Full model including lean mass shown with additional test for age*fat mass interaction:
Yij = β0 + Xij1β1 + Xij2β2 + Xij3β3 + Xij4β4 + Xij5β5 + Xij6β6 + Xij7β7 + Ai0 + Ai1age + eij
Yij = bone measurement for subject i at the jth measurement visit
β =coefficient for fixed effect
Xij1-3 = time as fixed effect (age, age2, age3
Xij4= fat mass
Xij5= limb length
Xij6 = lean mass
Xij7 = age*fat mass interaction
Ai0= overall tendency of participant i to differ from other participants (random intercept)
Ai1age= differential growth for participant i (random slope for linear growth)
eij = error in measurement visit j for participant i
Study Objective 2 (primary objective)
To determine whether greater pre-pubertal adiposity is significantly associated with subsequent age of maturation and adolescent bone strength. Pearson correlation coefficients were used to assess the relationship between BMI at age 8 and subsequent age of PHV. Next, differences in age of PHV were compared among healthy weight, overweight, and obese girls and boys using generalized linear models with p-values adjusted for multiple comparison. The overweight and obese groups were next combined (OW) to increase sample sizes for comparison with healthy-weight (HW) participants. Differences in bone strength from age 11 to 17 in OW compared with HW (reference group) girls and boys were estimated by adding the adiposity group variable to Model 1 using chronological age (centered age, age at a measurement visit – grand mean of age) as the time variable and then repeated using biological age in Model 1 and 2, as described above. In addition, models included a variable for change in fat mass from baseline to the final visit.
Model fitting for Study Objectives 1 and 2
Models of the longitudinal bone-fat relationship and of adiposity group strength differences were evaluated using Akaike's Information Criterion (AIC) goodness-of-fit statistic. The AIC was used to select the structure of the variance-covariance matrices of the between and within subject variances. The unstructured covariance matrix was selected for the random effects covariance matrix based on the AIC statistic for each model. The random error (within person or residual covariance matrix) covariance matrix was also evaluated, but models utilizing both the random effects and random error covariance matrices indicated the random error (residual covariance matrix) structure either would not converge or the model fit was not improved based on the AIC statistic.
Evaluation of Missing Data
Models were refit using imputed data to evaluate the effects of missing pQCT bone scan data. Bone data missing due to poor scan quality (i.e., movement or incorrect reference line placement) were imputed using the Markov Chain Monte Carlo (MCMC) method and missing data were considered missing at random. The frequency of pQCT measurements this included in girls and boys, respectively, was 10.4 and 12.2% at age 11, 8.2 and 10.2% at age 13, 6.2 and 6.1% at age 15, and 2.5 and 0.7% at age 17. Five imputed datasets were created using the SAS MI procedure and analyzed as described above. Results from the five datasets were combined to produce parameter estimates (SAS MIANALYZE procedure).
Results
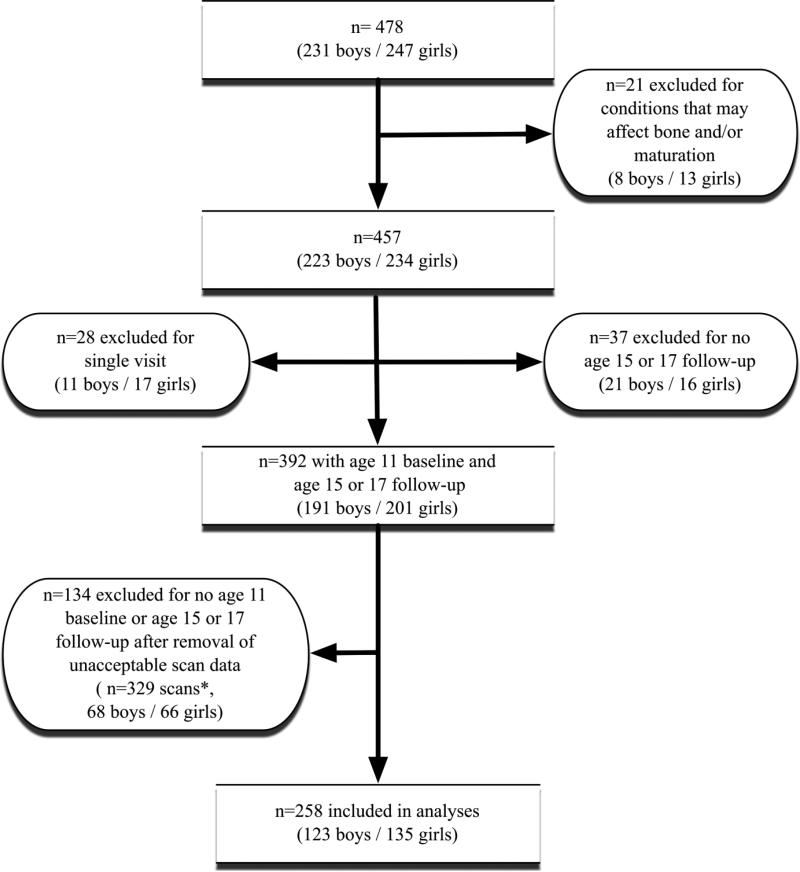
Out of a total of 478 participants, twenty-one were excluded from the present analyses due to medical conditions that could affect bone development or age of maturation, including a BMI ≤5th percentile for age and sex at baseline (n=13), type 1 diabetes (n=3), ulcerative colitis (n=2), cerebral palsy with left hemiparesis (n=1), lupus erythematosus (n=1) and premature adrenarche (n=1). As shown in Figure 1, 28 participants (11 boys and 17 girls) who attended only a single bone measurement visit, as well as 37 participants (21 boys and 16 girls) without an age 15 or age 17 follow-up visit, were also excluded from analyses. In addition, 329 pQCT scans were excluded from analyses due to unacceptable movement or reference line placement. This resulted in an additional exclusion of 68 boys and 66 girls who no longer had a minimum of two visits, an age 11 baseline visit and an age 15 or age 17 follow-up visit. Girls excluded at baseline for lack of follow-up (n=33) did not differ from those included (n=135) with respect to visit age, age of PHV, height, body composition or bone variables. Boys excluded at baseline for lack of follow-up (n=32) had a slightly younger age of PHV (mean±SD=13.3±0.7 vs 13.7±0.7 years, p=0.005), had greater lean mass (mean±SD=34.4±6.7 vs. 31.5±5.3 kg, p=0.012) and greater polar strength strain index (pSSI) at the tibia (mean±SD=1094.7±247.6 vs. 997.6±241.2 mm3, p=0.048) compared with those included in analyses (n=123).
Figure 1.
Participant inclusion diagram.
*Missing scan data due to movement or incorrect reference line placement. Data missing due to scan quality were imputed in additional analyses.
Sex-specific characteristics of eligible participants are shown in Table 1 by measurement visit. Lean mass and height were greater in boys than girls, reaching statistical significance beginning at the age 13 and age 15 measurement visits, respectively. At all visits, arm lean mass was greater in boys than girls while percentage body fat was greater in girls than boys. A larger percentage of girls had achieved estimated age of PHV at age 11 and 13 than boys. No other significant differences were noted.
Table 1.
Participant Characteristics by Sex and Measurement Visit (mean±SD or percentage)
| Measure | Girls | Boys | ||||||
|---|---|---|---|---|---|---|---|---|
| Age 11 n=135 |
Age 13 n=100 |
Age 15 n=109 |
Age 17 n=108 |
Age 11 n=123 |
Age 13 n=93 |
Age 15 n=98 |
Age 17 n=89 |
|
| Age (years) | 11.2±0.3 | 13.2±0.4 | 15.3±0.3 | 17.5±0.4 | 11.2±0.3 | 13.3±0.4 | 15.4±0.3 | 17.5±0.4 |
| Overweight/Obese (% yes) | 31.9/14.8 | 25.3/13.1 | 24.1/13.9 | 26.7/11.4 | 39.0/13.8 | 36.6/14.0 | 29.9/11.3 | 33.0/11.4 |
| Percentage Fat Mass (%) | 28.4±7.5* | 26.4±7.5* | 27.6±7.1* | 29.8±7.1 | 24.8±7.6 | 21.9±8.7 | 17.8±6.3 | 17.9±6.9 |
| Arm Lean Mass (kg) | 1.5±0.3* | 1.9±0.4* | 2.0±0.3* | 2.0±0.4* | 1.6±0.3 | 2.3±0.5 | 3.2±0.6 | 3.5±0.6 |
| Lean Mass (kg) | 30.4±5.9 | 37.4±6.2* | 41.6±6.0* | 43.5±7.2* | 31.2±5.3 | 42.1±7.1 | 54.5±8.4 | 60.9±8.1 |
| Height (cm) | 148.9±6.8 | 160.6±5.9 | 164.4±5.9* | 165.6±6.2* | 148.4±7.3 | 162.3±8.7 | 175.0±7.7 | 178.8±7.3 |
| Physical Activity Level (1-5)** | 2.8±0.7 | 2.6±0.7 | 2.4±0.7 | 2.3±0.7 | 2.9±0.7 | 2.8±0.8 | 2.6±0.8 | 2.5±0.9 |
| % Achieved Physical Maturity*** | 17.8* | 98.0* | 100 | 100 | 0 | 25.8 | 98.0 | 100 |
p-values <0.05 for girls vs. boys
higher values indicate higher activity levels
physical maturity defined as the age of peak height velocity
Longitudinal Relationship between Fat Mass and Bone Development
Results from the linear mixed model analyses of the associations between fat mass and bone parameters showed that the random intercept (participant divergence from average baseline bone parameter) was statistically significant for all bone parameters in girls and boys (data not shown). However, the slope parameter, indicating the individual rate of growth was not significant for radial tBMD (parameter estimate±SE=0.002±0.009, p=0.830), radial cBMC (0.003±0.002, p=0.166), radial CT (−0.003±0.006, p=0.652) or tibial PC in girls (0.004±0.003, p=0.087). In boys, the slope parameter was not significant for tibial PC (0.002±0.002, p=0.236) or radial tBMD (0.011±0.008, p=0.170). When random intercept and slope were included in the models for radial BSI in girls and boys as well as cBMD in boys, the variance component for slope was estimated to be zero, so final models for these outcomes did not include random slope. These findings provided support for inclusion of the individual-specific intercepts and slopes for most of the bone outcome models.
Results are presented as standardized beta±standard error (SE) unless otherwise specified. For girls, linear mixed model analyses of the associations between fat mass and bone parameters (Model 1) revealed that higher fat mass was associated with larger PC (0.08±0.03, p=0.002) and greater pSSI (0.08±0.03, p=0.008) at the radius (Table 2). At the tibia, higher fat mass was associated with greater tBMD (0.13±0.04, p=0.001) and cBMC (0.10±0.03, p<0.001), greater bone strength (BSI 0.15±0.04, p=0.001; pSSI (0.08±0.02, p<0.001) as well as larger PC (0.08±0.02, p<0.001) and CT (0.10±0.04, p=0.013). There was also a positive association between fat mass and Z (0.18±0.03, p<0.001) at the hip. These relationships were no longer significant after adjustment for variability in lean mass (Model 2). In addition, the relationship became significantly negative for radial CT (−0.11±0.04, p=0.013) and tibial BSI (−0.09±0.05, p=0.047). There was a significant interaction effect between age and fat mass on the relationship between fat mass and CT (main effect=−0.20±0.05, p<0.001, interaction=0.03±0.009, p=0.003) at the radius. This indicated that the association between fat mass and radial CT increased with age.
Table 2.
Sex-Specific Linear Mixed Model Estimates (standardized parameter estimate±SE) of the Association between Fat Mass and Bone Development from Age 11-17 Years
| Girls | |||
|---|---|---|---|
| Bone Measurement | *Model 1 | **Model 2 | |
| Fat mass | Fat Mass | Lean Mass | |
| DXA - Hip | |||
| Z | 0.18±0.03a | −0.02±0.04 | 0.55±0.07a |
| pQCT - Radius | |||
| Trabecular BMD | 0.05±0.06 | −0.01±0.06 | 0.18±0.06c |
| BSI | 0.06±0.06 | −0.08±0.06 | 0.43±0.06a |
| Cortical BMD | −0.03±0.03 | 0.02±0.03 | −0.14±0.03a |
| Cortical BMC | 0.03±0.03 | −0.03±0.03 | 0.20±0.03a |
| Periosteal Circumference | 0.08±0.03b | 0.02±0.03 | 0.22±0.03a |
| Endosteal Circumference | 0.05±0.02 | 0.06±0.02c | −0.07±0.03c |
| Cortical Thickness | −0.07±0.04 | −0.11±0.04c | 0.13±0.05c |
| pSSI | 0.08±0.03c | 0.01±0.03 | 0.24±0.03a |
| pQCT - Tibia | |||
| Trabecular BMD | 0.13±0.04c | −0.001±0.045 | 0.42±0.07a |
| BSI | 0.15±0.04b | −0.09±0.05c | 0.71±0.07a |
| Cortical BMD | −0.01±0.03 | 0.02±0.03 | −0.08±0.05 |
| Cortical BMC | 0.10±0.03b | −0.04±0.03 | 0.50±0.04a |
| Periosteal Circumference | 0.08±0.02b | −0.01±0.03 | 0.31±0.04a |
| Endosteal Circumference | 0.001±0.030 | 0.05±0.03 | −0.16±0.05c |
| Cortical Thickness | 0.10±0.04c | −0.08±0.04 | 0.56±0.06a |
| pSSI | 0.08±0.02b | −0.03±0.03 | 0.37±0.04a |
| Boys | |||
| DXA - Hip | |||
| Z | 0.03±0.02 | −0.05±0.02c | 0.76±0.07a |
| pQCT - Radius | |||
| Trabecular BMD | 0.002±0.05 | −0.02±0.05 | 0.74±0.10a |
| BSI | −0.002±0.04 | −0.04±0.04 | 1.02±0.07a |
| Cortical BMD | −0.02±0.04 | −0.01±0.04 | −0.33±0.08a |
| Cortical BMC | −0.06±0.02b | −0.06±0.02b | 0.39±0.04a |
| Periosteal Circumference | −0.03±0.02 | −0.04±0.02c | 0.39±0.03a |
| Endosteal Circumference | 0.07±0.02b | 0.08±0.02b | −0.26±0.05a |
| Cortical Thickness | −0.12±0.03b | −0.12±0.03b | 0.38±0.07a |
| pSSI | −0.04±0.02c | −0.03±0.02 | 0.40±0.04a |
| pQCT - Tibia | |||
| Trabecular BMD | −0.01±0.04 | −0.06±0.04 | 0.61±0.09a |
| BSI | 0.02±0.04 | −0.04±0.03 | 0.77±0.08a |
| Cortical BMD | −0.08±0.04b | −0.07±0.04 | −0.03±0.09 |
| Cortical BMC | 0.03±0.02 | −0.02±0.02 | 0.64±0.05a |
| Periosteal Circumference | 0.05±0.02c | 0.02±0.02 | 0.41±0.04a |
| Endosteal Circumference | 0.04±0.03 | 0.05±0.03 | −0.29±0.07a |
| Cortical Thickness | 0.01±0.03 | −0.04±0.03 | 0.68±0.07a |
| pSSI | 0.03±0.02 | 0.003±0.016 | 0.49±0.04a |
Z: narrow neck section modulus, BSI: bone strength index, pSSI: polar strength-strain index
Model 1: adjusted for biological age and limb length
Model 2: Model 1 further adjusted for lean mass
p-values:
p<0.0001
p<0.001
p<0.05
For boys, greater fat mass (Model 1) was associated with lower cBMC (−0.06±0.02, p<0.001), smaller CT (−0.12±0.03, p<0.001) and lower pSSI (−0.04±0.02, p=0.026) at the radius (Table 2). At the tibia, greater fat mass was associated with significantly lower cBMD (−0.08±0.04, p=0.034) but larger PC (0.05±0.02, p=0.012 Following adjustment for lean mass (Model 2), higher fat mass remained significantly associated with lower cBMC (−0.06±0.02, p<0.001) and smaller CT (−0.12±0.03, p<0.001) at the radius while a negative association with radial PC (−0.04±0.02, p=0.037), and hip Z (−0.05±0.02, p=0.009) became significant. There were significant interaction effects between age and fat mass for radial cBMC (main effect=−0.06±0.02, p<0.001, interaction=−0.013±0.004, p=0.001) and pSSI (main effect =−0.04±0.02, p=0.025, interaction=−0.009±0.004, p=0.025) and for tibial PC (main effect=0.04±0.02, p=0.041, interaction=−0.012±0.004, p=0.006). This indicated that strength of the associations between fat mass and radial pSSI and tibial PC decreased with age.
Analyses including imputed pQCT bone data
The same analyses repeated with the addition of imputed bone data revealed similar Model 2 results as those presented in Table 2. However, the negative associations at the radius between fat mass and CT in girls and PC in boys were no longer significant. At the tibia in boys, there were newly significant negative associations between fat mass and tBMD (−0.17±0.04, p<0.001), BSI (−0.17±0.04, p<0.001) and CT (−0.12±0.05, p=0.017). There was also a significant biological age*fat mass interaction effect for tibial BSI (main effect=−0.15±0.03, p<0.001; interaction=−0.03±0.01, p=0.017).
Relationship between Greater Adiposity and Subsequent Age of Peak Height Velocity
There was a significant negative correlation between BMI at age 8 and subsequent estimated age of PHV in girls (r=−0.61, p<0.001). There was also a significant, but much smaller correlation between age 8 BMI and estimated age of PHV in boys (r=−0.23, p=0.012). Obese girls had a significantly younger estimated age of PHV (mean±SD=11.1±0.5 years) compared with healthy weight (12.0±0.4 years, p<0.001) and overweight girls (11.5±0.5 years, p=0.010). Obese boys had a significantly younger estimated age of PHV (mean±SD=13.3±0.7 years) compared with the healthy weight (13.8±0.7 years, p=0.012) but not overweight boys (13.6±0.6 years, p=0.156). To increase sample sizes for comparison of bone strength among adiposity groups, the overweight and obese groups were combined (OW) and compared with healthy weight (HW) girls and boys. As shown in Table 3, OW compared with HW girls and boys had significantly greater lean, fat and total mass (all p<0.05) at all measurement visits, but similar physical activity levels (all p>0.05).
Table 3.
Characteristics of Healthy Weight (HW) versus Overweight/Obese (OW) Girls and Boys by Measurement Visit (mean±SD)
| Girls | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age 11 | Age 13 | Age 15 | Age 17 | |||||
| HW | OW | HW | OW | HW | OW | HW | OW | |
| N=91 | N=39 | N=66 | N=29 | N=74 | N=31 | N=74 | N=30 | |
| Age (years) | 11.2±0.3 | 11.3±0.3 | 13.3±0.4 | 13.2±0.3 | 15.3±0.3 | 15.3±0.4 | 17.5±0.4 | 17.5±0.4 |
| BMI | 47.5±25.0a | 92.8±6.7 | 46.7±23.3a | 90.0±9.2 | 48.6±23.7a | 85.1±15.4 | 50.8±24.7a | 85.9±17.2 |
| Percentile Height (cm) | 147.3±6.6b | 152.1±6.3 | 159.8±5.6 | 162.3±6.5 | 164.0±5.8 | 165.1±6.3 | 164.9±6.4 | 167.0±5.8 |
| Arm Lean Mass (kg) | 1.4±0.2a | 1.8±0.3 | 1.8±0.3a | 2.2±0.4 | 1.9±0.2a | 2.3±0.3 | 1.9±0.3a | 2.3±0.4 |
| Total Lean Mass (kg) | 27.9±0.4a | 36.1±0.5 | 35.8±0.4a | 43.8±0.7 | 39.3±0.4a | 46.8±0.6 | 40.9±0.5a | 49.9±0.8 |
| Total Fat Mass (kg) | 9.6±3.4a | 21.0±7.8 | 11.1±3.8a | 23.1±8.4 | 13.6±4.5a | 24.5±9.2 | 15.9±5.4a | 29.5±12.1 |
| Total Mass (kg) | 38.6±6.5a | 58.5±12.1 | 48.5±6.8a | 68.8±13.6 | 54.7±7.2a | 73.1±14.2 | 58.9±9.2a | 81.9±18.8 |
| Percentage Fat Mass (%) | 25.1±5.5a | 35.9±6.3 | 23.2±5.2a | 33.7±6.5 | 25.2±5.6a | 33.3±6.9 | 27.4±5.5a | 35.8±7.5 |
| Physical Activity Level (1-5)* | 2.8±0.7 | 2.7±0.7 | 2.6±0.8 | 2.6±0.5 | 2.5±0.8 | 2.3±0.7 | 2.3±0.8 | 2.3±0.7 |
| % Achieved Physical Maturity** | 7.7%a | 43.6% | 97.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% |
| Boys | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age 11 | Age 13 | Age 15 | Age 17 | |||||
| HW | OW | HW | OW | HW | OW | HW | OW | |
| N=78 | N=37 | N=60 | N=27 | N=62 | N=29 | N=57 | N=25 | |
| Age (years) | 11.3±0.3 | 11.2±0.3 | 13.3±0.4 | 13.2±0.3 | 15.4±0.3 | 15.4±0.4 | 17.6±0.4 | 17.5±0.4 |
| BMI | 57.7±26.0a | 90.1±9.4 | 57.6±28.1a | 89.1±9.6 | 58.1±24.9a | 85.4±14.5 | 58.5±27.2a | 87.2±13.9 |
| Percentile Height (cm) | 147.9±6.9 | 150.0±8.6 | 162.7±9.2 | 161.4±8.2 | 174.8±7.4 | 175.5±8.9 | 178.5±6.6 | 179.9±8.9 |
| Arm Lean Mass (kg) | 1.6±0.3a | 1.8±0.4 | 2.3±0.5 | 2.4±0.5 | 3.0±0.5b | 3.5±0.7 | 3.3±0.5a | 4.0±0.6 |
| Total Lean Mass (kg) | 30.0±0.4a | 35.3±0.6 | 40.9±0.7c | 44.5±0.6 | 52.0±0.7a | 60.1±0.9 | 58.2±0.7a | 67.2±0.8 |
| Total Fat Mass (kg) | 9.0±3.9a | 16.1±7.0 | 10.4±5.5a | 18.1±7.8 | 10.6±4.4b | 16.7±7.9 | 12.1±6.2b | 20.2±10.3 |
| Total Mass (kg) | 40.3±6.8a | 52.8±12.2 | 52.9±10.3a | 64.2±11.1 | 64.7±9.1a | 79.4±14.7 | 73.2±10.6a | 90.7±16.1 |
| Percentage Fat mass (%) | 22.5±6.4a | 30.3±7.1 | 19.6±7.8a | 28.1±8.3 | 16.6±5.5c | 21.1±7.2 | 16.6±6.0b | 22.1±7.9 |
| Physical Activity Level (1-5)* | 3.0±0.7 | 2.9±0.7 | 2.7±0.8 | 3.0±08 | 2.5±0.8 | 2.8±0.7 | 2.4±0.9 | 2.7±0.9 |
| % Achieved Physical Maturity** | 0.0% | 0.0% | 28.3% | 18.5% | 98.4% | 96.6% | 100.0% | 100.0% |
a higher number indicates greater physical activity
physical maturity defined as the age of peak height velocity
p<0.0001
p<0.001
p<0.05
The Relationship between Greater Adiposity and Subsequent Bone Strength
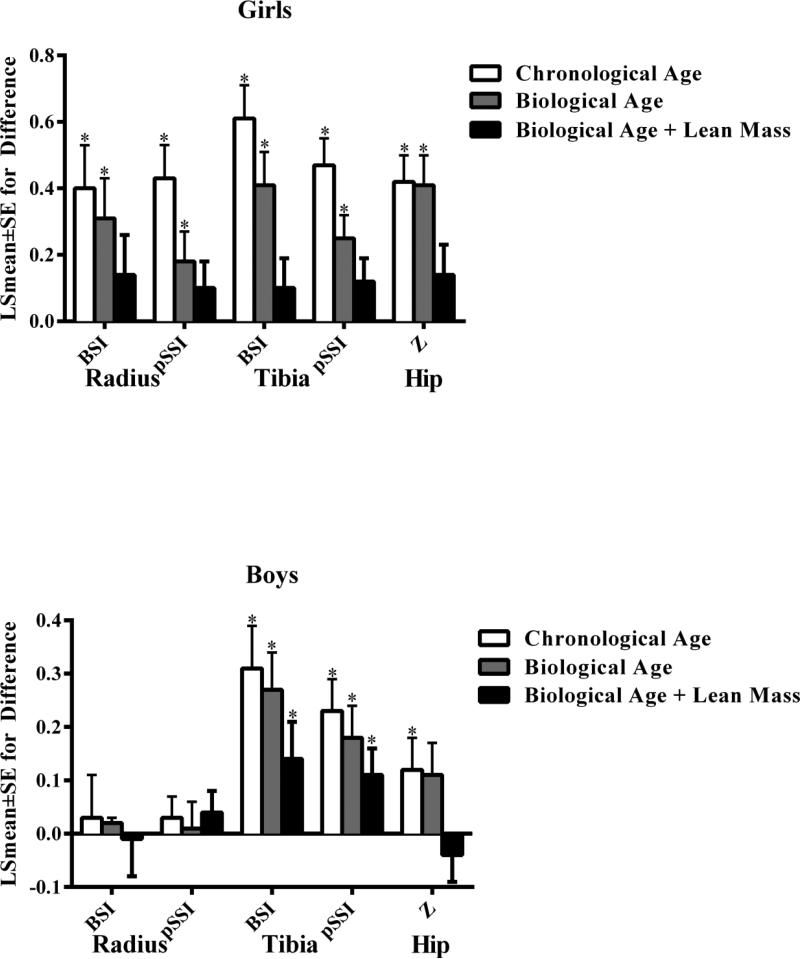
In linear mixed models including chronological age, OW compared with HW girls (reference group) had significantly higher metaphyseal (BSI) and diaphyseal (pSSI) bone strength at the radius (BSI: 0.40±0.13 p=0.002; pSSI=0.43±0.10, p<0.001) and tibia (BSI=0.61±0.10, p<0.001; SSI=0.47±0.08, p<0.001) as well as higher femoral neck bone strength (Z=0.42±0.08, p<0.001). These results are shown in Figure 2 with HW as the reference group. These between-group differences were significant at the tibia (BSI=0.31±0.08, p<0.001; pSSI=0.23±0.06, p<0.001) and femoral neck (Z=0.12±0.06, p=0.047), but not the radius in boys (BSI=0.03±0.08, p=0.686; pSSI=0.03±0.04, p=0.423).
Figure 2.
Differences (LSmean±SE) in bone strength in overweight versus healthy-weight (reference group) girls (top) and boys (bottom).
White bar: Model includes chronological age, limb length and change in fat mass between the first and last measurement visit
Black bar: Model substitutes biological age in place of chronological age
Grey bar: Model including biological age with addition of lean mass
BSI: bone strength index
pSSI: polar strength-strain index
Z: section modulus
*p<0.05
When analyses were repeated using biological age to account for differences in maturation level (Figure 2), group differences were reduced compared with the previous model at the radius (BSI=0.31±0.12, p=0.013; pSSI=0.18±0.09, p=0.037) and tibia (BSI=0.41±0.10, p<0.001; pSSI=0.25±0.07, p<0.001) with little difference at the femoral neck (Z=0.41±0.09, p<0.001) in girls. In boys, differences were slightly reduced at the radius (BSI=0.02±0.01, p=0.819; pSSI=0.01±0.05, p=0.881),tibia (BSI=0.27±0.07, p<0.001; pSSI=0.18±0.06, p=0.003) and femoral neck (Z=0.11±0.06, p=0.053). Following adjustment for lean mass, there were no longer significant between group differences in girls at the radius (BSI=0.14±0.12, p=0.256; pSSI=0.10±0.08, p=0.202), tibia (BSI=0.10±0.09, p=0.277; pSSI=0.12±0.07, p=0.081) or femoral neck (Z=0.14±0.09, p=0.124). In boys, parameter estimates were reduced at the femoral neck (Z=−0.04±0.05, p=0.473) and tibia following adjustment for lean mass (BSI=0.14±0.07, p=0.043; pSSI=0.11±0.05, p=0.032) with little change at the radius (BSI=−0.01±0.07, p=0.842; pSSI=0.04±0.04, p=0.332).
Discussion
This study sought to evaluate the bone-fat relationship during adolescence and determine whether greater, pre-pubertal adiposity is associated with subsequent timing of maturation and adolescent bone strength. We found approximately 5-6% greater diaphyseal bone strength at the radius and tibia and 11% greater femoral neck strength in OW compared with HW girls due to both earlier maturation and greater lean mass. However, OW compared with HW boys had approximately 5% greater diaphyseal bone strength at the tibia and 3% greater femoral neck strength due to greater lean mass but no bone strength differences were present at the radius. These sex- and site-specific differences in the relationships between greater, total adiposity and bone strength during adolescence suggest potentially different mechanisms could underlie these associations.
Sex-Specific Differences
Our hypothesis that greater adiposity would be associated with earlier subsequent maturation and greater bone strength in girls was partially supported by our findings. We found a significantly earlier age of maturation in OW compared with HW girls, which partially accounted for greater, subsequent bone strength. This is consistent with studies that showed obesity associated with earlier maturation,(4-7) greater bone mineral accrual in early versus average maturing females by 20 years of age,(11) timing of puberty negatively associated with bone density at the time of skeletal maturity,(10) and higher percent body fat associated with earlier maturation and greater bone size and density by age 16.(36) Specifically, we found greater bone strength in OW compared with HW girls and that both biological age and lean mass accounted for these differences. This is also consistent with results of our analyses of the relationships between fat mass, lean mass and bone parameters evaluated as continuous variables in models adjusted for biological age. Those results revealed significant, positive associations between bone strength and lean, but not fat mass.
Our hypothesis that greater pre-pubertal adiposity would be associated with a later subsequent age of maturation and lower bone strength was not supported by our findings. We found slightly earlier rather than later maturation in OW compared with HW boys, which did not, however, appear to contribute to differences in bone strength. In fact, parameter estimates for differences in bone strength between OW and HW boys modeled with either chronological or biological age were similar. These findings are in concordance with studies that separately showed earlier maturation associated with higher BMI(4) and no differences in bone mineral accrual over a period of 15 years among early, normal and late maturing boys.(11) However, this could also indicate that the statistically significant earlier age of maturation in OW compared with HW boys (~6 months) might not have been sufficient enough to influence bone strength. The parameter estimates for greater bone strength in OW compared with HW boys at the tibia were, instead, lessened after accounting for lean mass. This is consistent with the mechanostat theory where muscle strength rather than body weight is considered the primary driver of bone adaptations.(37) Despite both earlier maturation and greater lean mass in OW compared with HW boys in our study, radius bone strength did not differ between groups. This result, along with findings from our analyses of the associations between fat mass and bone parameters as continuous variables, suggest a potentially negative bone-fat relationship at the mid-shaft of the non-weight-bearing radius in boys. Specifically, we found that greater fat mass was associated with thinner cortical bone and lower diaphyseal bone strength at the radius and that parameter estimates for these associations were relatively unchanged after adjustment for lean mass. Taken together, these findings suggest a possible influence from greater adiposity on the timing of maturation and adolescent bone strength in girls that is not present, to the same extent, in boys.
Site-Specific Findings
A lack of differences in OW vs HW radius bone strength in boys, despite earlier maturation and greater lean mass, lends support to observations that the bone-fat relationship might vary according to adipose tissue type. First, visceral fat secretes inflammatory factors(38) and has been negatively associated with femoral bone strength.(39) Secondly, excess fat is generally gained in the central region in males. As a result, a potentially greater amount of visceral fat in OW boys could, to some extent, contribute to observed sex differences in our findings. Finally, bone strength findings at the radius might also be more likely to reflect influence from biochemical and/or genetic factors not evident at the hip or tibia. At weight-bearing sites such as the hip or tibia, greater muscle strength necessary to move higher mass in overweight individuals during habitual loading could, hypothetically, offset other factors that might otherwise influence bone strength. Future investigations are needed to evaluate potential mechanisms that might help explain these site-specific findings in boys.
Implications
It is unclear whether the significantly greater bone strength noted in this study in OW compared with HW girls and boys, is advantageous and translates into sufficient bone strength for body mass. It has previously been suggested that bone is adequately adapted to lean, but not fat or overall mass, in overweight individuals. In a cross-sectional study that compared HW and OW individuals ages 4-20 years, Petit and colleagues found indices of proximal femur geometry similar after accounting for lean mass.(40) In another study, Wetzsteon and colleagues found baseline pSSI and change over 16 months significantly higher in OW compared with HW children ages 9-11 years.(41) However, there were no differences in pSSI relative to lean mass, and the pSSI increase was associated with an increase in lean but not fat mass. In both studies, bone parameters (geometry or strength) were found to be low in relation to fat or overall mass. Still others have found bone area and mineral accrual low for body weight in OW compared with HW children.(42) In our study, OW compared with HW girls and boys, respectively, had 51.6% and 26.9% higher total mass, 119.6% and 78.0% higher fat mass but only 17.7% and 12.5% higher arm lean mass and 29.4% and 17.7% higher total lean mass at baseline. Thus, the bone strength differences in our study might not be sufficiently large enough to compensate for higher mass in OW individuals. Furthermore, a lack of bone strength differences at the forearm in boys could indicate that bone strength might be insufficient to accommodate higher fall-related loading with higher body weight in OW boys.
Potential Limitations
Results from our study should be interpreted in light of the following considerations. First, estimated age of PHV, though an objectively measured marker of maturation in both boys and girls, is a surrogate indicator of the rapid changes in hormones and growth factors that were not directly measured in this study. In addition, Tanner stage estimates were not available for the entire time-period covered by the study. While self-assessment of Tanner stage was obtained beginning at age 11 in our study, 86.7% of girls reported a Tanner stage of 2 or greater at that visit which reduced the utility of that measure to meaningfully differentiate between girls in maturation level at baseline and subsequent visits. Furthermore, we did not obtain annual measurements necessary to determine age of PHV and instead this was estimated from visits taking place every two years. It should be noted that height measurements were taken every 2 years rather than annually in our cohort. As such, the age of PHV was estimated from a series of cross-sectional estimates taking into account the relative precision of estimates at different stages of growth. Another potential limitation to this study was the use of baseline adiposity status (age 8) to define greater adiposity cut-offs, when adiposity status could have changed during the study. However, adiposity level tracked well across visits, with correlations among adiposity measurements ranging from r=0.80 to 0.90 (p<0.001) and analyses were adjusted for changes in fat mass. It should also be noted that, in our study, girls and boys were not directly compared in analyses. Finally, the study cohort is comprised of primarily Midwestern, Caucasian Americans with relatively high socioeconomic status and results from this study are not generalizable to other populations.
Strengths
There are several strengths of this study that contributed to a better understanding of the relationship between greater adiposity and subsequent maturation and bone strength. First, data were prospectively collected and analyses accounted for individual variation in both baseline bone measures and rate of change, as well as changes in lean and fat mass and body size during growth. Furthermore, there is a wide range of body composition represented among participants and our cohort is not limited to athletes. Other studies have evaluated the association between timing of maturation and bone parameters(10,11) or the relationships among adiposity, maturation and bone parameters in girls alone(43) or in both girls and boys.(36) However, because those studies either utilized DXA, a measurement technique influenced by body size, did not evaluate males and/or estimates of bone strength, findings from our study broaden the literature on the relationship between childhood obesity and bone strength. To our knowledge, this is the first longitudinal study to prospectively evaluate the relationship between greater pre-pubertal adiposity and subsequent maturation and bone strength during adolescence at both weight-bearing and non-weight-bearing sites by pQCT.
Conclusions
The results of this study suggest sex- and site-specific differences in the association between pre-pubertal fat mass and bone strength from age 11 to 17 years. OW compared with HW girls had an earlier age of maturation and greater lean mass, which accounted for both weight-bearing and non-weight-bearing bone strength differences. Despite earlier maturation and greater lean mass in OW compared with HW boys, there were no differences in bone strength at the non-weight-bearing radius, suggesting a negative influence from greater adiposity. At the tibia and femoral neck, differences in bone strength between adiposity groups in boys were reduced after accounting for lean mass. These findings suggest that greater adiposity is a stronger contributor to subsequent maturation and bone strength in girls than boys, and appears to negatively influence non-weight-bearing cortical bone strength in boys. Future studies are needed to determine whether these findings are sustained in adulthood.
Acknowledgments
Special thanks to the Iowa Bone Development Study participants and staff for their time, hard work and dedication to this project.
This study was supported by NIDCR(R01-DE09551and DE12101), GCRCP(M01-RR00059) and NCRR(UL1TR000442).
Footnotes
Disclosures
The authors have no disclosures.
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jbmr.2809]
-
1)substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: all authors
-
2)participated in drafting the manuscript or revising it critically for important intellectual content: all authors
-
3)approved the final version of the submitted manuscript: all authors
-
4)agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: NG
References
- 1.Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in children and adolescents: United States, 2005-2008. NCHS Data Brief. 2010(51):1–8. [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360(9331):473–82. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 4.Aksglaede L, Juul A, Olsen LW, Sorensen TI. Age at puberty and the emerging obesity epidemic. PLoS One. 2009;4(12):e8450. doi: 10.1371/journal.pone.0008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aksglaede L, Sorensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics. 2009;123(5):e932–9. doi: 10.1542/peds.2008-2491. [DOI] [PubMed] [Google Scholar]
- 6.Walvoord EC. The timing of puberty: is it changing? Does it matter? J Adolesc Health. 2010;47(5):433–9. doi: 10.1016/j.jadohealth.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Biro FM, Greenspan LC, Galvez MP. Puberty in girls of the 21st century. J Pediatr Adolesc Gynecol. 2012;25(5):289–94. doi: 10.1016/j.jpag.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. 2002;110(5):903–10. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]
- 9.Lee JM, Kaciroti N, Appugliese D, Corwyn RF, Bradley RH, Lumeng JC. Body mass index and timing of pubertal initiation in boys. Arch Pediatr Adolesc Med. 2010;164(2):139–44. doi: 10.1001/archpediatrics.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilsanz V, Chalfant J, Kalkwarf H, et al. Age at onset of puberty predicts bone mass in young adulthood. J Pediatr. 2011;158(1):100–5. 5, e1–2. doi: 10.1016/j.jpeds.2010.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackowski SA, Erlandson MC, Mirwald RL, et al. Effect of maturational timing on bone mineral content accrual from childhood to adulthood: evidence from 15 years of longitudinal data. Bone. 2011;48(5):1178–85. doi: 10.1016/j.bone.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Kindblom JM, Lorentzon M, Norjavaara E, et al. Pubertal timing predicts previous fractures and BMD in young adult men: the GOOD study. J Bone Miner Res. 2006;21(5):790–5. doi: 10.1359/jbmr.020602. [DOI] [PubMed] [Google Scholar]
- 13.Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. Deleterious effect of late menarche on distal tibia microstructure in healthy 20-year-old and premenopausal middle-aged women. J Bone Miner Res. 2009;24(1):144–52. doi: 10.1359/jbmr.080815. [DOI] [PubMed] [Google Scholar]
- 14.Rauch F, Klein K, Allolio B, Schonau E. Age at menarche and cortical bone geometry in premenopausal women. Bone. 1999;25(1):69–73. doi: 10.1016/s8756-3282(99)00104-0. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein JS, Neer RM, Biller BM, Crawford JD, Klibanski A. Osteopenia in men with a history of delayed puberty. N Engl J Med. 1992;326(9):600–4. doi: 10.1056/NEJM199202273260904. [DOI] [PubMed] [Google Scholar]
- 16.Belanger C, Luu-The V, Dupont P, Tchernof A. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm Metab Res. 2002;34(11-12):737–45. doi: 10.1055/s-2002-38265. [DOI] [PubMed] [Google Scholar]
- 17.Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140(3):399–410. doi: 10.1530/REP-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20(4):535–82. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- 19.Hammoud AO, Gibson M, Peterson CM, Hamilton BD, Carrell DT. Obesity and male reproductive potential. J Androl. 2006;27(5):619–26. doi: 10.2164/jandrol.106.000125. [DOI] [PubMed] [Google Scholar]
- 20.Clayton PE, Gill MS, Hall CM, Tillmann V, Whatmore AJ, Price DA. Serum leptin through childhood and adolescence. Clin Endocrinol (Oxf) 1997;46(6):727–33. doi: 10.1046/j.1365-2265.1997.2081026.x. [DOI] [PubMed] [Google Scholar]
- 21.Clayton PE, Trueman JA. Leptin and puberty. Arch Dis Child. 2000;83(1):1–4. doi: 10.1136/adc.83.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blum WF, Englaro P, Hanitsch S, et al. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab. 1997;82(9):2904–10. doi: 10.1210/jcem.82.9.4251. [DOI] [PubMed] [Google Scholar]
- 23.Bass SL, Saxon L, Daly RM, et al. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res. 2002;17(12):2274–80. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- 24.Warden SJ, Mantila Roosa SM, Kersh ME, et al. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci U S A. 2014;111(14):5337–42. doi: 10.1073/pnas.1321605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry YM, Fatayerji D, Eastell R. Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: relative contributions of bone size and volumetric bone mineral density. Osteoporos Int. 2004;15(4):263–73. doi: 10.1007/s00198-003-1542-9. [DOI] [PubMed] [Google Scholar]
- 26.Heaney RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporos Int. 2000;11(12):985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 27.Janz KF, Broffitt B, Levy SM. Validation evidence for the Netherlands physical activity questionnaire for young children: the Iowa bone development study. Res Q Exerc Sport. 2005;76(3):363–9. doi: 10.1080/02701367.2005.10599308. [DOI] [PubMed] [Google Scholar]
- 28.Levy SM, Kiritsy MC, Slager SL, Warren JJ. Patterns of dietary fluoride supplement use during infancy. J Public Health Dent. 1998;58(3):228–33. doi: 10.1111/j.1752-7325.1998.tb02998.x. [DOI] [PubMed] [Google Scholar]
- 29.Ramamurthi K, Ahmad O, Engelke K, et al. An in vivo comparison of hip structure analysis (HSA) with measurements obtained by QCT. Osteoporos Int. 2012;23(2):543–51. doi: 10.1007/s00198-011-1578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kontulainen SA, Johnston JD, Liu D, Leung C, Oxland TR, McKay HA. Strength indices from pQCT imaging predict up to 85% of variance in bone failure properties at tibial epiphysis and diaphysis. J Musculoskelet Neuronal Interact. 2008;8(4):401–9. [PubMed] [Google Scholar]
- 31.Weatherholt AM, Avin KG, Hurd AL, et al. Peripheral Quantitative Computed Tomography Predicts Humeral Diaphysis Torsional Mechanical Properties With Good Short-Term Precision. J Clin Densitom. 2015;18(4):551–9. doi: 10.1016/j.jocd.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34(4):689–94. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: preliminary evidence for the Physical Activity Questionnaire for Older Children. Med Sci Sports Exerc. 1997;29(10):1344–9. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Kowalski KC, Crocker PR, Kowalski NP. Convergent validity of the physical activity questionnaire for adolescents. Pediatric Exercise Science. 1997;9(4):342–52. [Google Scholar]
- 35.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 36.Streeter AJ, Hosking J, Metcalf BS, Jeffery AN, Voss LD, Wilkin TJ. Body fat in children does not adversely influence bone development: a 7-year longitudinal study (EarlyBird 18). Pediatr Obes. 2013 doi: 10.1111/j.2047-6310.2012.00126.x. [DOI] [PubMed] [Google Scholar]
- 37.Frost HM. Bone's mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275(2):1081–101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 38.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 39.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94(9):3387–93. doi: 10.1210/jc.2008-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petit MA, Beck TJ, Shults J, Zemel BS, Foster BJ, Leonard MB. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone. 2005;36(3):568–76. doi: 10.1016/j.bone.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Wetzsteon RJ, Petit MA, Macdonald HM, Hughes JM, Beck TJ, McKay HA. Bone structure and volumetric BMD in overweight children: a longitudinal study. J Bone Miner Res. 2008;23(12):1946–53. doi: 10.1359/jbmr.080810. [DOI] [PubMed] [Google Scholar]
- 42.Goulding A, Taylor RW, Jones IE, McAuley KA, Manning PJ, Williams SM. Overweight and obese children have low bone mass and area for their weight. Int J Obes Relat Metab Disord. 2000;24(5):627–32. doi: 10.1038/sj.ijo.0801207. [DOI] [PubMed] [Google Scholar]
- 43.Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. Pubertal timing and body mass index gain from birth to maturity in relation with femoral neck BMD and distal tibia microstructure in healthy female subjects. Osteoporos Int. 2011;22(10):2689–98. doi: 10.1007/s00198-011-1531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]