Abstract
BACKGROUND
Chronic rhinosinusitis with nasal polyps (CRSwNP) is an inflammatory condition of the nasal passage and paranasal sinuses characterized by Th2 biased inflammation with elevated levels of BAFF, B-lymphocytes, and immunoglobulins. Since high levels of BAFF are associated with autoimmune diseases, we assessed for evidence of autoimmunity in patients with CRS.
OBJECTIVES
The objective of this study was to investigate for the presence of autoantibodies in sinonasal tissue from patients with CRS.
METHODS
Standardized nasal tissue specimens were collected from patients with CRS and control subjects and assayed for immunoglobulin production, autoantibody levels, tissue distribution of immunoglobulins and binding potential of antibodies in nasal tissue using a multiplexed autoantibody microarray, ELISA and immunofluoresence.
RESULTS
Elevated levels of several specific autoantibodies were found in nasal polyp tissue in comparison with control tissue and inflamed tissue from non-polypoid CRS (CRSsNP) (p<0.05). In particular, nuclear-targeted autoantibodies such as anti-dsDNA IgG and IgA antibodies were found at elevated levels in nasal polyps (p<0.05) and particularly in nasal polyps from patients requiring revision surgery for recurrence. Direct immunofluorescence staining demonstrated diffuse epithelial and sub-epithelial deposition of IgG and increased numbers of IgA secreting plasma cells not seen in control nasal tissue.
CONCLUSIONS
Autoantibodies, particularly those against nuclear antigens, are present at locally elevated levels in nasal polyps. The presence of autoantibodies suggests that the microenvironment of a nasal polyp promotes the expansion of self-reactive B-cell clones. While the pathogenicity of these antibodies remains to be elucidated, the presence of elevated anti-dsDNA antibodies is associated with a clinically more aggressive form of CRSwNP requiring repeated surgery.
Keywords: Chronic Rhinosinusitis, Sinusitis, Nasal Polyps, Autoimmunity, Autoantibodies, Biomarker
Introduction
Chronic rhinosinusitis (CRS) is a clinical syndrome associated with persistent inflammation of nasal and paranasal sinuses mucosa. This definition encompasses the two most common variants of this disease - a form with nasal polyps (CRSwNP) and a non-polypoid form (CRSsNP) that have clinically and morphologically different characteristics1. Historically, CRSsNP was considered as an incompletely treated case of acute rhinosinusitis resulting in chronic infection. CRSwNP was considered a distinct, non-infectious disorder of unclear etiology, perhaps related to atopy2. While both forms of disease utilize surgery as a modality for improving paranasal sinus drainage and relieving patient symptoms, medical therapy for both forms utilizes antibiotics and corticosteroids3-5. Treatment success for either form of CRS is variable with no currently established molecular predictors to guide choice of therapy or predict outcome.
Emerging research from our laboratory highlights a potentially important pathogenic role for B-lymphocytes in the inflammation associated with CRS. We have shown that the B-cell activating factor of the TNF family (BAFF, also called BLys, or TNFSF13B) is highly elevated in nasal polyp tissue in CRSwNP in comparison to CRSsNP, control and unaffected tissue in CRSwNP6. We found that BAFF is produced by epithelial cells and could be induced by stimulation with several cytokines and innate immune activators7. BAFF is a potent stimulator of B-cell proliferation and class switching in B-cells, and mice over-expressing BAFF manifest systemic autoimmunity8, 9. In addition to BAFF, we have also found nasal polyps contain elevated levels of the cytokine IL6 and chemokines such as BLC (CXCL13) and SDF-1a which are known to play a role in B-cell recruitment and plasma cell differentiation10, 11. We have proposed that these findings may account for the increased levels of IgA and IgG present in nasal polyp tissue, germinal-center like pseudofollicles and consistently high numbers of B cells and plasma cells10, 12. At present, the nature of the antigen specificity of these B cells and their roles in pathogenesis of nasal polyposis remain unclear13.
The nature and specificity of the immunoglobulins found in nasal polyps has not been explored in detail. In 1974, Bass et al. examined the distribution of the different immunoglobulin subtypes in nasal polyps and found no significant IgM, IgA was found in numerous plasma cells in the sub-epitelial and periglandular regions of nasal polyps while IgG was found diffusely deposited thoughout the stroma14. Due to the proposed atopic etiology of nasal polyposis, the vast majority of the research focuses on IgE. Gevaert et al demonstrated a polyclonal IgE hyperglobulinemia along with elevated levels of specific IgE against aeroallergens and staphylococcus enterotoxins compared to serum12. Similarly, a more recent study by Sabirov et al. demonstrated the presence of elevated levels of IgE against Alternaria alternata relative to serum15. Tellingly, similar elevations in local specific IgG and IgA suggested that local excess production of immunoglobulin was not isolated to the IgE isotype. We hypothesized that the local mucosal inflammatory microenvironment and chronic inflammation associated with CRSwNP was conducive to the expansion of autoreactive B-cell clones that may play a role in perpetuating inflammation. In this study, we examined nasal tissue for the presence of class switched autoantibodies as evidence that such phenomena might exist in CRS.
Methods
Patients and tissue samples
Patients with CRS were recruited from a tertiary care allergy and otolaryngology practice at the Northwestern University Feinberg School of Medicine. CRS was defined by the criteria established by the American Academy of Otolaryngology-Head and Neck Surgery Chronic Rhinosinusitis Task Force16. All CRS patients had failed a standardized course of medical therapy and were consented for tissue collection at the time of surgery. Specimens from control subjects were obtained during endoscopic skull base tumor excisions, intranasal procedures for obstructive sleep apnea and facial fracture repairs for patients without a history of sinonasal inflammation. Further details on the clinical selection criteria and tissues collected for the subjects enrolled in this study can be found in the online repository. None of the patients enrolled in this study had a history of autoimmune disease. Informed consent was obtained from all patients prior to surgery and these protocols were reviewed and approved by the Northwestern University institutional review board (IRB). Details of the subjects’ characteristics and sample types are described in Table I.
Table 1.
Subject Characteristics
| CRS with Nasal polyps | CRS without Nasal Polyps | Control Tissue | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 44 | (26M/18F) | 25 | (10M/15F) | 22 | (17M/5F) | |||||
| Average Age | 42 | 44 | 47 | ||||||||
| Atopy | 29 | 5 | 0 | ||||||||
| Asthma | 24 | 2 | 0 | ||||||||
| Prior Nasal Surgery | 12 | 6 | 0 | ||||||||
| Average Follow Up | 13.5m | 13m | N/A | ||||||||
| Nasal Polyp Grade |
1.84 | ||||||||||
| Tissue Type | Array | EIA | IF/H2 | Array | EIA | Array | EIA | IF/H2 | |||
| Inferior Turbinate | 20 | 5 | 20 | 25 | 25 | 22 | 10 | 22 | |||
| Uncinate Process | 18 | 18 | 10 | 10 | 4 | 4 | 2/2 | ||||
| Polyp | 37 | 10 | 37 | 8/6 | N/A | N/A | |||||
F, Female; M, Male; IF, Immunofluorescence; m, Months; H2, Hep2
Preparation of polyp/sinus tissue extracts
Detergent extracts of sinonasal surgical tissue samples were prepared as described previously, further details can be found in the Online Repository7, 17.
Assessment of autoantibodies in nasal polyp tissue using autoantigen microarrays
In order to comprehensively screen nasal polyps for the presence of autoantibodies, an autoantigen proteomic microarray was utilized to simultaneously assess samples for autoantibodies using an array of 63 known autoantigens18. Additional details on the preparation of samples for analysis on the autoantibody microarrays can be found in the online repository. We defined a measurable spot if the fluorescent intensity was >50 and statistical analysis was performed if there were 5 or more samples with measurable spots for the particular autoantibody25. A positive result was defined as any antigen with a p-value of <0.05 using a Mann-Whitney U test comparison of polyp extract to control inferior turbinate extract.
Enzyme-linked Immunoassay approach confirming the presence of anti-dsDNA autoantibodies in nasal polyps
Anti-dsDNA quantitative IgA and IgG EIAs (Alpco Diagnostics, Salem NH) were used to assay an expanded set of nasal tissue extracts from control subjects and patients with CRSwNP and CRSsNP. Since the EIA protocols for this kit were established for examining serum, modifications in the initial dilution and subsequent use of the serum-based standard curves were necessary for examining nasal tissue extract. Briefly, nasal tissue extracts were appropriately diluted for analysis within the diagnostic EIAs linear range and analyzed according to the manufacturer’s instructions. In general, tissue extracts from control and CRSsNP subjects were diluted at 1:10 with diluent while polyp tissue frequently required dilutions ranging from 1:50 to 1:500 in some cases in order to obtain results within the linear range of the EIA. The provided reference serum provided was used to generate a standard curve but the results were linearly adjusted to account for differences in dilution factor. The results were mathematically normalized to the extracts total protein content for comparison between groups. The color intensity was measured with a Bio-Rad Spectrophotometer Model 680 Microplate Reader (Bio-Rad Laboratories, Hercules, CA) with associated software applied to the sandwich enzyme immunoassay technique. The detection range for these EIAs was 30IU/ml to 150,000 IU/ml.
Immunofluorescence
Preparation of nasal tissue for immunofluorescence was performed as previously described17. Briefly, paraffin embedded tissue was rehydrated, treated with antigen retrieval unmasking reagent (Vector Laboratories, Burlingame, Calif), rinsed and blocked. For direct immunofluorescence, tissue sections were then incubated with a rabbit antihuman IgG polyclonal Ab (Dako, Carpinteria, CA) at a 1:2000 dilution. All sections were rinsed and then incubated with a secondary Alexafluor 488 conjugated goat anti-rabbit IgG(Molecular Probes) at a 1:500 dilution for 1 hour at room temperature. A modified indirect fluorescence assay was designed using control uncinate process tissue sections treated with 40 μg total protein/ml of control uncinate or nasal polyp tissue extract for 6 hours prior to incubation with the rabbit anti-human IgG polyclonal Ab. Additionally, a Hep-2 based assay was performed after treating immobilized Hep-2 cells (MBL-Woburn MA) with 200 μg total protein/ml of nasal tissue extracts (n=8) containing a range of anti-dsDNA autoantibody levels. Following treatment with the nasal tissue extracts, the samples were treated with conjugate, mounted and analyzed using a fluorescent microscope. Nuclear staining intensity was semi-quantitatively scored by two observers (0-4) blinded to the anti-dsDNA content in each sample. Details of the image acquisition can be found in the online repository.
Statistics
Unless otherwise specified, all results were normalized to the total protein content as determined by the BCA protein assay. Comparisons were performed using the Kruskal-Wallis one-way ANOVA since the distribution of autoantibodies in tissue was non-Gaussian and a post-hoc Dunn’s test was used to evaluate the binary comparisons. Binary comparisons were carried out using a Mann-Whitney U test. For frequency analysis, we defined a sample to have an elevated anti-dsDNA level at 3-standard deviations above the mean autoantibody measurements obtained from the tissue derived from control patients. Linear regression was performed and R-squared values calculated for correlations. All analyses were performed using software obtained from GraphPad Prism (La Jolla, CA). A 2-tailed p-value of less than 0.05 was considered statistically significant.
Results
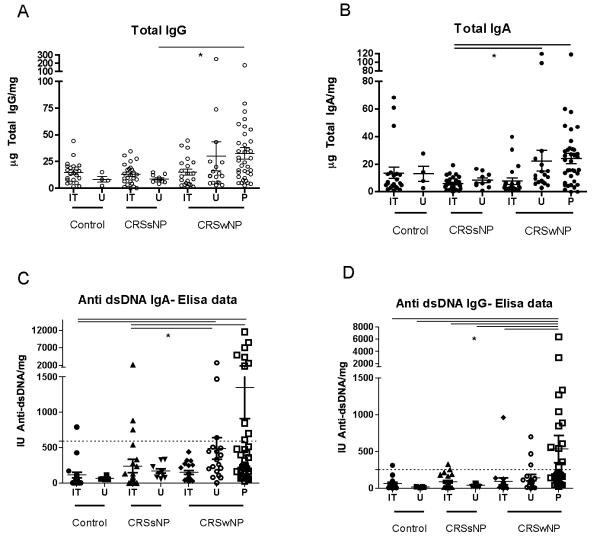
Clinical and demographic characteristics of the patients enrolled in this study are shown in Table 1. Twelve of the patients with CRSwNP and six of the patients with CRSsNP were undergoing revision nasal surgery. Total IgA levels and IgG levels were elevated in nasal polyp tissue extract relative to control nasal tissue extracts (See Figure 1A and 1B respectively).
Figure 1.
Total and anti-dsDNA levels in nasal tissue. A. Total IgG; B. Total IgA. Anti-dsDNA levels of C. IgA and D. IgG subtypes. The dashed line represents the mean +3SD of the levels of autoantibodies found in control nasal tissue. All analyses had a significant Kruskal-Wallis test, * signifies a significant post-hoc Dunn’s test. Results are normalized to total protein levels.
Analysis of autoantibody microarray results
The results of the autoantibody microarray demonstrated measurable IgG and IgA autoantibody levels against 25 of the 62 antigens and 24 of the 62 antigens respectively. Statistical analysis revealed that relative to control nasal tissue, statistically significant elevations of several autoantibodies were found in nasal polyp tissue. There were no autoantibodies that were elevated in control nasal tissue extracts relative to nasal polyp extracts. Results of the statistically significant comparisons for both the IgG and IgA isotype antibodies are depicted in Table 2 along with several representative non-significant differences. There were more positive results for IgG autoantibodies than IgA. Revealingly, we observed that a large number of elevated autoantibodies were reactive against nuclear antigens. Interestingly, the elevated levels of autoantibodies were confined to nasal polyp tissue extract and were not observed in the inferior turbinates from patients with CRSwNP.
Table 2.
Summary of the autoantibody microarray results with all statistically significant differences and selected autoantibodies with non-significant differences.
| IgG Autoantibodies | ||||
|---|---|---|---|---|
| Name | Target | Median (CRSwNP Polyp) |
Median (Normal IT) |
p |
|
| ||||
| Chromatin | Chromatin | 90.0 | 17.8 | 0.0007 |
| B2-microglobulin | Cell Surface | 1306.0 | 321.0 | 0.0012 |
| H3 | Histone | 67.5 | 8.3 | 0.0015 |
| Laminin | ECM | 29.5 | 1.5 | 0.0019 |
| dsDNA | DNA | 177.0 | 38.3 | 0.0025 |
| Heparan Sulfate | Cell Surface/ECM | 105.0 | 28.0 | 0.0025 |
| Matrigel | ECM | 71.5 | 14.3 | 0.0025 |
| H1 | Histone | 70.5 | 17.3 | 0.0039 |
| TPO | Thyroid Peroxidase | 207.5 | 49.0 | 0.006 |
| BPAG | Hemidesmosome | 138.5 | 47.3 | 0.0075 |
| Fibrinogen IV | Basement Membrane | 83.0 | 28.3 | 0.0075 |
| Human rFc-gamma-RIIA | Cell Surface | 187.7 | 99.2 | 0.0137 |
| E.coli | Bacteria | 189.0 | 96.8 | 0.0167 |
| Thyroglobulin | Thyroiglobulin | 106.0 | 56.0 | 0.0242 |
| Ribo phosphoprotein | Nuclear Protein | 109.5 | 38.3 | 0.0346 |
| PCNA | Nucleoplasm | 46.0 | 13.5 | 0.0486 |
| rHuman PDGFR sR | Cell Surface | 324.0 | 279.5 | 0.0486 |
| IgA Autoantibodies | ||||
|---|---|---|---|---|
| Name | Target | Median (CRSwNP Polyp) |
Median (Normal IT) |
p |
|
| ||||
| dsDNA | DNA | 122.5 | 35.75 | 0.0092 |
| Chromatin | Chromatin | 84.5 | 17.25 | 0.0167 |
| H1 | H1 | 48 | 13.25 | 0.0221 |
| IgG Autoantibodies | ||||
|---|---|---|---|---|
| Name | Target | Median (CRSwNP Polyp) |
Median (Control IT) |
p |
|
| ||||
| Chromatin | Chromatin | 90.0 | 17.8 | 0.0007 |
| B2-microglobulin | Cell Surface | 1306.0 | 321.0 | 0.0012 |
| H3 | Histone | 67.5 | 8.3 | 0.0015 |
| Laminin | ECM | 29.5 | 1.5 | 0.0019 |
| dsDNA | DNA | 177.0 | 38.3 | 0.0025 |
| Heparan Sulfate | Cell Surface/ECM | 105.0 | 28.0 | 0.0025 |
| Matrigel | ECM | 71.5 | 14.3 | 0.0025 |
| H1 | Histone | 70.5 | 17.3 | 0.0039 |
| TPO | Thyroid Peroxidase | 207.5 | 49.0 | 0.006 |
| BPAG | Hemidesmosome | 138.5 | 47.3 | 0.0075 |
| Fibrinogen IV | Basement Membrane | 83.0 | 28.3 | 0.0075 |
| Human rFc-gamma-RIIA | Cell Surface | 187.7 | 99.2 | 0.0137 |
| E.coli | Bacteria | 189.0 | 96.8 | 0.0167 |
| Thyroglobulin | Thyroiglobulin | 106.0 | 56.0 | 0.0242 |
| Ribo phosphoprotein | Nuclear Protein | 109.5 | 38.3 | 0.0346 |
| PCNA | Nucleoplasm | 46.0 | 13.5 | 0.0486 |
| rHuman PDGFR sR | Cell Surface | 324.0 | 279.5 | 0.0486 |
| IgA Autoantibodies | ||||
|---|---|---|---|---|
| Name | Target | Median (CRSwNP Polyp) |
Median (Control IT) |
p |
|
| ||||
| dsDNA | DNA | 122.5 | 35.75 | 0.0092 |
| Chromatin | Chromatin | 84.5 | 17.25 | 0.0167 |
| H1 | H1 | 48 | 13.25 | 0.0221 |
p<0.05 considered significant
EIA based analysis of anti-dsDNA levels in nasal tissue
We next attempted to confirm the elevations of both the IgA and IgG autoantibodies focusing on anti-dsDNA in light of the well-described pathogenic potential of anti-dsDNA antibodies in lupus and the high sensitivity of the commercially based assays. A quantitative EIA utilizing recombinant human DNA to specifically assay for dsDNA (Alpco Diagnostics, Salem, NH) binding was utilized. An expanded set of tissue comprising extracts of nasal polyps (P) and nasal tissue from the inferior turbinates (IT) and uncinate processes (U) obtained from patients with CRSwNP, CRSsNP and controls was used. These results demonstrate a 6 and 7-fold higher level of anti-dsDNA IgA (Figure 1C) and IgG (Figure 1D) antibodies respectively in nasal polyp tissue when compared with control inferior turbinate tissue. Multi-group comparisons revealed statistically significant elevations of anti-dsDNA IgG and IgA antibodies with significant post-hoc pairwise comparisons of nasal polyps to nasal tissue from patients with CRSsNP and controls. Within CRSwNP, statistically significant elevations were found in some nasal polyp tissue relative to the inferior turbinate but not the uncinate processes. The latter observation is consistent with clinical observations that the uncinate process is more closely involved with the inflammatory process in CRSwNP.
Correlation of IgG anti-dsDNA to Total IgG levels
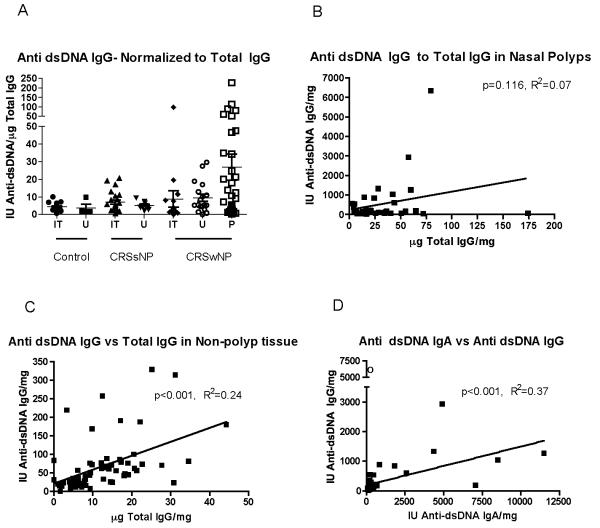
The lack of measurable autoantibodies in over half the antigens on the microarray makes it unlikely that these observations are secondary to a non-specific binding from elevated levels of IgG and IgA in nasal polyp extract. To assess whether the elevated levels of anti-dsDNA antibodies were due to non-specific elevated immunoglobulin levels, the anti-dsDNA IgG results were normalized to total IgG levels (Figure 2A). This revealed a statistically significant multi-group comparison although post-hoc testing did not reveal a specific binary comparison that was statistically significant. Within nasal polyp tissue, there was no correlation between anti-dsDNA autoantibody levels and total IgG (Figure 2B). However, data derived from tissue samples from non-CRSwNP patients showed a positive correlation between anti-dsDNA IgG autoantibody levels and Total IgG (Figure 2C). These results suggest that the production of anti-dsDNA IgG antibodies in nasal polyps was independent of total IgG levels. There was a positive correlation between the anti-dsDNA IgG and anti-dsDNA IgA levels (Figure 2D) suggesting that the production of the two isotypes is driven by related processes.
Figure 2.
Correlation of anti-dsDNA antibody levels. A. The level of Anti-dsDNA IgG normalized to Total IgG levels. This analysis had a significant Kruskal-Wallis test but post-hoc testing was non-significant. B. Anti-dsDNA IgG levels did not correlate with Total IgG levels in nasal polyp tissue. C. Conversely, anti-dsDNA IgG levels correlated with Total IgG in nasal tissue from control and CRSsNP patients. D. Anti-dsDNA IgG and IgA levels in nasal polyps are positively correlated. In one patient (marked by a “o”), no detectable total IgA was measured in the patient’s nasal tissue raising the possibility of an IgA deficiency and we excluded this sample from the analysis in D. Except for A., all results are normalized to the total protein concentration.
Correlation of CRSwNP disease activity with locally elevated levels of anti-dsDNA
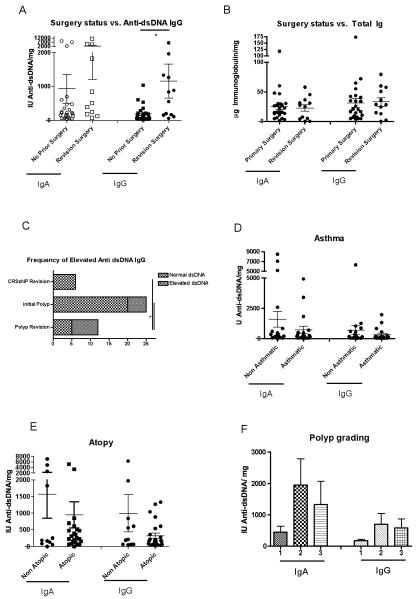
We then correlated anti-dsDNA autoantibody levels with the clinical parameters collected on our patients. We found that elevated levels of the IgG anti-dsDNA autoantibodies were found at higher levels (Figure 3A) and more frequently (Figure 3C) in patients with more aggressive clinical course requiring revision surgery. There was a trend toward higher levels of anti-dsDNA antibodies in higher-grade nasal polyps (Figure 3F). None of the patients who were receiving revision surgery for CRSsNP, had elevated levels of anti-dsDNA IgG antibodies (Figure 3C). Anti-dsDNA autoantibodies were not affected by asthma status or atopic status (Figure 3D and 3E respectively). In contrast, aggressive nasal polyps requiring revision surgery was not correlated with total IgA and IgG levels (Figure 3B).
Figure 3.
Correlation between anti-dsDNA levels in nasal polyps and clinical parameters. A. The anti-dsDNA IgG in recurrent nasal polyps was elevated compared to those undergoing initial nasal polyp surgery. B. Total immunoglobulin levels were not correlated with surgical status. C. Frequency of elevated anti-dsDNA IgG in different subgroups. Anti-dsDNA antibodies are not differentially found in D. asthmatics and E. atopic patients. F. Higher grade polyps have higher anti-dsDNA levels. All results are normalized to total protein levels.
Direct and Indirect Immunoflorescence
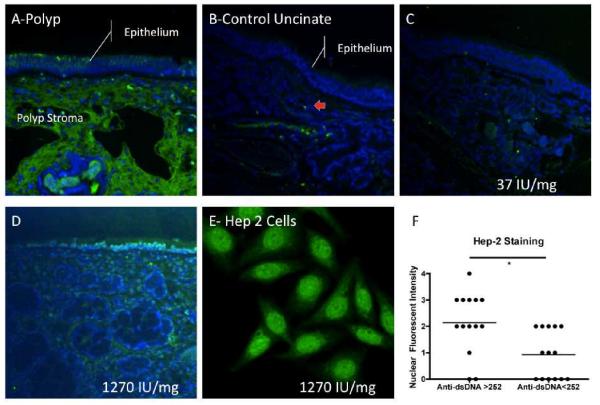
Using direct immunofluorescence of nasal polyps, we frequently observed extensive deposition of IgG within the stroma of the nasal polyps. Most IgG was deposited extracellularly with some regions enriched in IgG containing plasma cells (not shown). In some samples, there was intraepithelial IgG deposition seen (Figure 4A). In contrast, control nasal tissue showed little extravascular IgG and few perivascular IgG secreting plasma cells (Figure 4B, plasma cell denoted by red arrow). In contrast, IgA in nasal polyps was largely confined to intracellular IgA in subepithelial and stromal plasma cells and within secretory cells within the glandular tissue (data not shown). Control tissue stained with isotype-controlled rabbit IgG showed no specific staining (data not shown).
Figure 4.
Immunofluorescence of nasal tissue. A. IgG direct immunofluorescence in a nasal polyp (green fluorescence) and B. control nasal uncinate tissue showing scattered plasma cells (red arrow). Indirect Immunofluorescence of control uncinate tissue incubated with: C. tissue extract from control patient and D. nasal polyp extract from a patient with elevated level of anti-dsDNA autoantibodies. Nuclei were counterstained with DAPI (blue fluorescence), IgG was labeled using anti-IgG (green fluorescence). Normalized anti-dsDNA IgG levels found in each of the tissue extracts (numbers in white). E. Hep-2 assay on nasal polyp extract from the same patient. F. Nuclear staining intensity of samples analyzed on Hep-2 assay.
Using a modified indirect immunofluorescence technique, we incubated sections of control uncinate process tissue with nasal tissue extract from a series of two control uncinate processes and eight nasal polyps with a range of anti-dsDNA IgG autoantibodies (range= 65 to 6332 U anti-dsDNA IgG /mg Total Protein) and examined the resulting sections using direct immunofluorescence. In this assay, if the autoreactive antibodies present in the nasal extract were predominantly anti-nuclear, a nuclear staining pattern would be anticipated, and the staining intensity would parallel the anti-dsDNA antibodies found in the tissue. The staining pattern when control uncinate tissue was incubated with control tissue extract was similar to those obtained from direct immunofluorescence of control uncinate tissue (Compare Figure 4B-direct immunofluorescence and Figure 4C-indirect immunofluorescence with control extract). In contrast, there was a specific nuclear staining pattern seen in some samples incubated with polyp extracts containing high levels of anti-dsDNA antibodies (Figure 4D). The Hep-2 based indirect immunofluorescence assay (Figure 4E) demonstrated anti-nuclear antibodies in some nasal polyp extracts. In this assay, heavy non-nuclear “spindle” staining was seen in addition to the nuclear staining being scored. This resulted in some difficulty in directly using the assay criteria established for scoring serum indirect immunofluorescence. However, examining only nuclear staining, nasal tissue extracts from samples with elevated anti-dsDNA had more intense nuclear staining (Figure 4F).
Discussion
Chronic rhinosinusitis (CRS) is a prevalent chronic inflammatory condition of the paranasal sinuses that affects approximately eight percent of the United States population. Recently, our laboratory demonstrated evidence for overproduction of BAFF and IL6 in nasal polyp tissue. Since both these cytokines are associated with autoimmunity, we sought to examine nasal polyps for evidence of autoreactive B-cells9, 19, 20. The recent development of an autoantigen microarray enabled us to simultaneously query nasal polyp tissue for the presence of multiple autoantibodies18. As our microarray results suggest, a polyclonal, class switched autoantibody response against nuclear components, thyroid antigens and some epithelial antigens is found in nasal polyps relative to control nasal tissue and inflamed tissue from CRSsNP (Table 2).
Given their central pathogenic role in systemic lupus erythematosus (SLE), we chose to confirm the positive result for the anti-dsDNA antibodies on commercially available EIAs. In our analysis of nasal tissues from patients with CRSwNP, highly elevated levels of autoantibodies are found locally within some nasal polyps and more modestly in the uncinate process when compared with the clinically unaffected inferior turbinates (Figure 1). Uncinate process tissue in CRSsNP, which is frequently involved in the chronic inflammation, showed no elevation in the levels of anti-dsDNA antibodies suggesting that chronic inflammation alone does not result in elevated autoantibodies. Futhermore, the anti-dsDNA antibody production in nasal polyps was disproportionately elevated in comparison with total immunoglobulin levels (Figure 2). Clinically, nasal polyps obtained from patients undergoing revision surgery for recurrence frequently had elevated anti-dsDNA IgG antibodies compared with nasal polyps obtained from primary surgery. A similar trend was seen for the anti-dsDNA IgA antibodies although this did not achieve statistical significance (Figure 3A). In contrast, there was no evidence for increased autoantibodies in patients with CRSsNP who had multiple revision surgeries, suggesting that multiple surgeries themselves are not the trigger of this response (Figure 3C). Elevated levels of anti-dsDNA antibodies were found in some polyp samples obtained at initial surgery and it would be interesting to evaluate if they had clinical evidence for recurrence on longitudinal follow up. It is known that 25-75% of CRSwNP patients experience recurrence depending on the technique and length of follow up21, 22 and a biomarker to predict outcome following surgery would be clinically valuable. Given that there are surgery naïve CRSwNP patients with autoantibody elevations and several CRSsNP patients who had previous nasal surgery without evidence of elevated levels of autoantibodies, the possible interpretation that surgical trauma triggers autoantibody production is unlikely.
In systemic lupus erythermatosus (SLE), the presence of B cell and T cell autoimmunity to the nucleosome, and its individual components namely native dsDNA and histones, are important in establishing a diagnosis and correlate with the severity of clinical parameters such as lupus nephritis and lupus associated cognitive impairment23-26. The proposed mechanisms by which anti-dsDNA antibodies induce nephritis include the formation of immune complexes with DNA/nucleosome components released from apoptotic cells and cross reactivity with components of the basement membrane27, 28. While the pathogenicity of the anti-dsDNA antibodies in CRSwNP has still not been established, our data suggest that the presence of anti-dsDNA antibodies has implications on clinical parameters independent of total immunoglobulin levels. Whether these autoantibodies are directly pathogenic and bind to nucleosomal components or represent an epiphenomenon of a more severe and persistent form of sinonasal inflammation remains to be determined. These results also raise the possibility that memory B-cells reactive to self-antigens may persist after surgical removal of the inflamed tissue and potentially play a role in triggering recurrent inflammation.
While anti-nuclear antibody response is considered a dominant feature in SLE31, other organ-specific autoimmune diseases such as Sjogren’s syndrome and psoriasis also have elevated anti-dsDNA antibodies32, 33. Experimental models suggest autoimmunity results from the initial activation of naïve T-cells through microbial molecular mimicry or superantigens and are subsequently amplified by the enhanced processing and presentation of autoantigens and bystander activation of lymphocytes in an inflamed site35, 36. In CRSwNP, there is evidence for increased local viral and bacterial colonization providing the context by which pathogen derived peptides may cross activate autoreactive T or B cells37-39. Furthermore, evidence for staphylococcus superantigen expansion with Vβ skewing of T cell populations exists in CRSwNP, providing another mechanism for non-selective activation of autoreactive T-cells39-41. Following activation of these autoreactive B and T cells, epitope spreading and proliferation of these autoreactive clones can occur, thus expanding B-cells producing specific immunoglobulins to foreign antigens such as aeroallergens and colonizing microbial bacteria. The generation of autoantibodies and specific antibodies to extrinsic antigens is frequently found in other chronic inflammatory diseases of the epithelial interface. For example, in IBD, elevated levels of autoantibodies to perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA) along with antibodies to gut microflora such as Escherichia coli and Gram-positive anaerobic rods are found42-44.
A limitation of this study was that we focused analysis to tissue specific autoantibodies largely due to limited availability of matching sets of serum and nasal lavage particularly in the revision cases in which the autoantibodies appear most elevated. It will be of value to perform a matched tissue, nasal lavage and serum study to clarify whether circulating systemic autoantibodies can be detected within a subset of patients with nasal polyps and whether these correlate with intranasal autoantibody levels. Given the lack of reports of associated autoimmune conditions46 and the relatively limited mucosal surface area involved in CRS, it is probably unlikely that circulating autoantibodes will be significantly elevated. In addition to self-reactive IgG autoantibodies, our data suggest that IgA autoantibodies are present within nasal polyps. Since IgA is a potent stimulator of eosinophil degranulation, it raises the possibility of secreted autoreactive IgA antibodies propagating inflammation to more distal portions of the airway47.
Currently, B-cells are increasingly recognized as important therapeutic targets in many autoimmune diseases. The importance of B-cells in linking innate and adaptive immunity, and their contribution toward long term immune memory is evident due to the success of rituximab in treating multiple autoimmune diseases previously thought to be T-cell driven processes48. Interestingly, clinical experience with rituximab therapy in autoimmune disease demonstrates that levels of autoantibodies such as anti-dsDNA decrease more than the total immunoglobulin levels following therapy49. These data suggest that autoantibodies are produced by shorter-lived CD20+ B-cell dependent plasma cells or plasmablasts. Additionally, an anti-BAFF agent, Belimumab, was recently approved as a novel treatment for SLE. Data from the clinical trials of Belimumab demonstrates that selective targeting of BAFF has clinical activity and reduces anti-dsDNA antibodies secondary to depletion of both naïve and transitional B-cells without affecting traditional memory B-cells. While we have much to learn about the anti-dsDNA producing B-cells in CRSwNP, their presence within the most therapeutically refractory patients provides new potential avenues for targeted therapy in this disease.
Clinical implications.
The presence of elevated levels of anti-dsDNA IgG antibodies is correlated with therapeutically refractory disease and may have important implications in diagnosis, prognosis and therapy for CRSwNP.
Capsule summary.
This article provides novel evidence that inflammatory processes within nasal polyps facilitate the activation and expansion of autoreactive B-cells. Our data also suggest that elevated levels of anti-nuclear antibodies correlates with clinically refractory nasal polyposis.
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest. This work was supported by NIH grants R01 HL068546, R01 HL078860 and R01 AI072570 as well as the Ernest S. Bazley Trust.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huvenne W, van Bruaene N, Zhang N, van Zele T, Patou J, Gevaert P, et al. Chronic rhinosinusitis with and without nasal polyps: what is the difference? Curr Allergy Asthma Rep. 2009;9:213–20. doi: 10.1007/s11882-009-0031-4. [DOI] [PubMed] [Google Scholar]
- 2.Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22:549–59. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hissaria P, Smith W, Wormald PJ, Taylor J, Vadas M, Gillis D, et al. Short course of systemic corticosteroids in sinonasal polyposis: a double-blind, randomized, placebo-controlled trial with evaluation of outcome measures. J Allergy Clin Immunol. 2006;118:128–33. doi: 10.1016/j.jaci.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Thomas M, Yawn BP, Price D, Lund V, Mullol J, Fokkens W. EPOS Primary Care Guidelines: European Position Paper on the Primary Care Diagnosis and Management of Rhinosinusitis and Nasal Polyps 2007 - a summary. Prim Care Respir J. 2008;17:79–89. doi: 10.3132/pcrj.2008.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Zele T, Gevaert P, Holtappels G, Beule A, Wormald PJ, Mayr S, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010;125:1069–76. doi: 10.1016/j.jaci.2010.02.020. e4. [DOI] [PubMed] [Google Scholar]
- 6.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–92. doi: 10.1016/j.jaci.2008.03.002. 92 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol. 2006;177:7164–72. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorn M, Lewis RH, Mumbey-Wafula A, Kantrowitz S, Spatz LA. BAFF overexpression promotes anti-dsDNA B-cell maturation and antibody secretion. Cell Immunol. 2010;261:9–22. doi: 10.1016/j.cellimm.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 10.Patadia M, Dixon J, Conley D, Chandra R, Peters A, Suh LA, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy. 2009;24:11–16. doi: 10.2500/ajra.2010.24.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters AT, Kato A, Zhang N, Conley DB, Suh L, Tancowny B, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2010;125:397–403. doi: 10.1016/j.jaci.2009.10.072. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–9. doi: 10.1111/j.1398-9995.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 13.Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:21–6. doi: 10.1097/MOO.0b013e3283350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bass RM, Potter EV, Barney PL. Immunofluorescent localization of immunoglobulins in nasal polyps. Arch Otolaryngol. 1974;99:446–8. doi: 10.1001/archotol.1974.00780030460012. [DOI] [PubMed] [Google Scholar]
- 15.Sabirov A, Hamilton RG, Jacobs JB, Hillman DE, Lebowitz RA, Watts JD. Role of local immunoglobulin E specific for Alternaria alternata in the pathogenesis of nasal polyposis. Laryngoscope. 2008;118:4–9. doi: 10.1097/MLG.0b013e3181567a7a. [DOI] [PubMed] [Google Scholar]
- 16.Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 17.Tieu DD, Peters AT, Carter RT, Suh L, Conley DB, Chandra R, et al. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125:667–75. doi: 10.1016/j.jaci.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li QZ, Xie C, Wu T, Mackay M, Aranow C, Putterman C, et al. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest. 2005;115:3428–39. doi: 10.1172/JCI23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosello S, Youinou P, Daridon C, Tolusso B, Bendaoud B, Pietrapertosa D, et al. Concentrations of BAFF correlate with autoantibody levels, clinical disease activity, and response to treatment in early rheumatoid arthritis. J Rheumatol. 2008;35:1256–64. [PubMed] [Google Scholar]
- 20.Kishimoto T. Interleukin-6 and its receptor in autoimmunity. J Autoimmun. 1992;5(Suppl A):123–32. doi: 10.1016/0896-8411(92)90027-n. [DOI] [PubMed] [Google Scholar]
- 21.Albu S, Tomescu E, Mexca Z, Nistor S, Necula S, Cozlean A. Recurrence rates in endonasal surgery for polyposis. Acta Otorhinolaryngol Belg. 2004;58:79–86. [PubMed] [Google Scholar]
- 22.Larsen K, Tos M. A long-term follow-up study of nasal polyp patients after simple polypectomies. Eur Arch Otorhinolaryngol. 1997;254(Suppl 1):S85–8. doi: 10.1007/BF02439732. [DOI] [PubMed] [Google Scholar]
- 23.Suenaga R, Abdou NI. Cationic and high affinity serum IgG anti-dsDNA antibodies in active lupus nephritis. Clin Exp Immunol. 1993;94:418–22. doi: 10.1111/j.1365-2249.1993.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein KA, Kahl LE, Balow JE, Lefkowith JB. Serologic markers of lupus nephritis in patients: use of a tissue-based ELISA and evidence for immunopathogenic heterogeneity. Clin Exp Immunol. 1994;98:60–5. doi: 10.1111/j.1365-2249.1994.tb06607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suenaga R, Abdou NI. Anti-(DNA-histone) antibodies in active lupus nephritis. J Rheumatol. 1996;23:279–84. [PubMed] [Google Scholar]
- 26.Kowal C, Degiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci U S A. 2006;103:19854–9. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deshmukh US, Bagavant H, Fu SM. Role of anti-DNA antibodies in the pathogenesis of lupus nephritis. Autoimmun Rev. 2006;5:414–8. doi: 10.1016/j.autrev.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Rekvig OP, Nossent JC. Anti-double-stranded DNA antibodies, nucleosomes, and systemic lupus erythematosus: a time for new paradigms? Arthritis Rheum. 2003;48:300–12. doi: 10.1002/art.10739. [DOI] [PubMed] [Google Scholar]
- 29.Schlosser RJ, Mulligan RM, Casey SE, Varela JC, Harvey RJ, Atkinson C. Alterations in gene expression of complement components in chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24:21–5. doi: 10.2500/ajra.2010.24.3399. [DOI] [PubMed] [Google Scholar]
- 30.Van Zele T, Coppieters F, Gevaert P, Holtappels G, Van Cauwenberge P, Bachert C. Local complement activation in nasal polyposis. Laryngoscope. 2009 doi: 10.1002/lary.20484. [DOI] [PubMed] [Google Scholar]
- 31.Smeenk RJ. Antinuclear antibodies: cause of disease or caused by disease? Rheumatology (Oxford) 2000;39:581–4. doi: 10.1093/rheumatology/39.6.581. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Singh U. Prevalence of autoantibodies in patients of psoriasis. J Clin Lab Anal. 2010;24:44–8. doi: 10.1002/jcla.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fauchais AL, Martel C, Gondran G, Lambert M, Launay D, Jauberteau MO, et al. Immunological profile in primary Sjogren syndrome: clinical significance, prognosis and long-term evolution to other auto-immune disease. Autoimmun Rev. 2010;9:595–9. doi: 10.1016/j.autrev.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Chen C, Nagy Z, Radic MZ, Hardy RR, Huszar D, Camper SA, et al. The site and stage of anti-DNA B-cell deletion. Nature. 1995;373:252–5. doi: 10.1038/373252a0. [DOI] [PubMed] [Google Scholar]
- 35.Wucherpfennig KW. Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest. 2001;108:1097–104. doi: 10.1172/JCI14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Ghelue M, Moens U, Bendiksen S, Rekvig OP. Autoimmunity to nucleosomes related to viral infection: a focus on hapten-carrier complex formation. J Autoimmun. 2003;20:171–82. doi: 10.1016/s0896-8411(02)00110-5. [DOI] [PubMed] [Google Scholar]
- 37.Zaravinos A, Bizakis J, Spandidos DA. Prevalence of human papilloma virus and human herpes virus types 1-7 in human nasal polyposis. J Med Virol. 2009;81:1613–9. doi: 10.1002/jmv.21534. [DOI] [PubMed] [Google Scholar]
- 38.Bendouah Z, Barbeau J, Hamad WA, Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg. 2006;134:991–6. doi: 10.1016/j.otohns.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Bachert C, Zhang N, van Zele T, Gevaert P, Patou J, van Cauwenberge P. Staphylococcus aureus enterotoxins as immune stimulants in chronic rhinosinusitis. Clin Allergy Immunol. 2007;20:163–75. [PubMed] [Google Scholar]
- 40.Conley DB, Tripathi A, Seiberling KA, Suh LA, Harris KE, Paniagua MC, et al. Superantigens and chronic rhinosinusitis II: analysis of T-cell receptor V beta domains in nasal polyps. Am J Rhinol. 2006;20:451–5. doi: 10.2500/ajr.2006.20.2880. [DOI] [PubMed] [Google Scholar]
- 41.Conley DB, Tripathi A, Seiberling KA, Schleimer RP, Suh LA, Harris K, et al. Superantigens and chronic rhinosinusitis: skewing of T-cell receptor V beta-distributions in polyp-derived CD4+ and CD8+ T cells. Am J Rhinol. 2006;20:534–9. doi: 10.2500/ajr.2006.20.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bossuyt X. Serologic markers in inflammatory bowel disease. Clin Chem. 2006;52:171–81. doi: 10.1373/clinchem.2005.058560. [DOI] [PubMed] [Google Scholar]
- 43.Brandtzaeg P, Carlsen HS, Halstensen TS. The B-cell system in inflammatory bowel disease. Adv Exp Med Biol. 2006;579:149–67. doi: 10.1007/0-387-33778-4_10. [DOI] [PubMed] [Google Scholar]
- 44.Young Y, Abreu MT. Advances in the pathogenesis of inflammatory bowel disease. Curr Gastroenterol Rep. 2006;8:470–7. doi: 10.1007/s11894-006-0037-1. [DOI] [PubMed] [Google Scholar]
- 45.Aoki S, Yaoita H, Kitajima Y. An elevated level of autoantibodies against 48- to 50- kd keratins in the serum of patients with psoriasis. J Invest Dermatol. 1989;92:179–83. doi: 10.1111/1523-1747.ep12276700. [DOI] [PubMed] [Google Scholar]
- 46.Chandra RK, Lin D, Tan B, Tudor RS, Conley DB, Peters AT, et al. Chronic rhinosinusitis in the setting of other chronic inflammatory diseases. Am J Otolaryngol. 2010 doi: 10.1016/j.amjoto.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abu-Ghazaleh RI, Fujisawa T, Mestecky J, Kyle RA, Gleich GJ. IgA-induced eosinophil degranulation. J Immunol. 1989;142:2393–400. [PubMed] [Google Scholar]
- 48.Dorner T, Radbruch A, Burmester GR. B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol. 2009;5:433–41. doi: 10.1038/nrrheum.2009.141. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida T, Mei H, Dorner T, Hiepe F, Radbruch A, Fillatreau S, et al. Memory B and memory plasma cells. Immunol Rev. 2010;237:117–39. doi: 10.1111/j.1600-065X.2010.00938.x. [DOI] [PubMed] [Google Scholar]