Abstract
Sleep has been proposed to be a physiological adaptation to conserve energy, but little research has examined this proposed function of sleep in humans. We quantified effects of sleep, sleep deprivation and recovery sleep on whole-body total daily energy expenditure (EE) and on EE during the habitual day and nighttime. We also determined effects of sleep stage during baseline and recovery sleep on EE. Seven healthy participants aged 22 ± 5 years (mean ± s.d.) maintained ∼8 h per night sleep schedules for 1 week before the study and consumed a weight-maintenance diet for 3 days prior to and during the laboratory protocol. Following a habituation night, subjects lived in a whole-room indirect calorimeter for 3 days. The first 24 h served as baseline – 16 h wakefulness, 8 h scheduled sleep – and this was followed by 40 h sleep deprivation and 8 h scheduled recovery sleep. Findings show that, compared to baseline, 24 h EE was significantly increased by ∼7% during the first 24 h of sleep deprivation and was significantly decreased by ∼5% during recovery, which included hours awake 25–40 and 8 h recovery sleep. During the night time, EE was significantly increased by ∼32% on the sleep deprivation night and significantly decreased by ∼4% during recovery sleep compared to baseline. Small differences in EE were observed among sleep stages, but wakefulness during the sleep episode was associated with increased energy expenditure. These findings provide support for the hypothesis that sleep conserves energy and that sleep deprivation increases total daily EE in humans.
Non-technical summary
One of the proposed functions of sleep is to conserve energy. We determined the amount of energy conserved by sleep in humans, how much more energy is expended when missing a night of sleep, and how much energy is conserved during recovery sleep. Findings support the hypothesis that a function of sleep is to conserve energy in humans. Sleep deprivation increased energy expenditure indicating that maintaining wakefulness under bed-rest conditions is energetically costly. Recovery sleep after sleep deprivation reduced energy use compared to baseline sleep suggesting that human metabolic physiology has the capacity to make adjustments to respond to the energetic cost of sleep deprivation. The finding that sleep deprivation increases energy expenditure should not be interpreted that sleep deprivation is a safe or effective strategy for weight loss as other studies have shown that chronic sleep deprivation is associated with impaired cognition and weight gain.
Introduction
One of the proposed functions of sleep is to conserve energy (Berger & Phillips, 1995). Energy expenditure (EE) is hypothesized to be lower during sleep versus wakefulness to reduce total daily energy needs. In support of this theory, sleeping metabolic rate has been reported to be lower than resting metabolic rate during wakefulness with estimated reductions in EE of 7 to 69% among different mammalian species (Toutain et al. 1977; White et al. 1985; Wiersma et al. 2005; Revell & Dunbar, 2007). In humans, it is well established that EE is lower following the onset of sleep and during the scheduled sleep episode when compared to pre-sleep wakefulness (Kreider et al. 1958; Robin et al. 1958; Buskirk et al. 1960; White et al. 1985; Fraser et al. 1989; Bonnet et al. 1991); however, the total daily energy conserved by sleep has yet to be quantified in humans. Therefore, the primary aim of the current study was to quantify for the first time the energy conserved during sleep, or conversely, the metabolic cost of missing one night of sleep in humans. Specifically, we compared total daily EE during a habitual day consisting of 16 h wakefulness and 8 h sleep to EE during 24 h of total sleep deprivation and hypothesized that sleep deprivation would significantly increase total daily EE. We also quantified for the first time the energy conserved during recovery sleep following total sleep deprivation. We hypothesized that during the habitual nighttime, sleep would significantly reduce EE compared to sleep deprivation and that recovery sleep would significantly decrease EE compared to a habitual night of sleep.
Significant differences in EE have been reported between sleep stages in some (Brebbia & Altshuler, 1965; Fontvieille et al. 1994) but not all studies (Webb & Hiestand, 1975; Haskell et al. 1981; White et al. 1985; Palca et al. 1986). Of those reporting differences in EE between sleep stages, one consistent finding was lower EE during deep slow-wave sleep (SWS). Therefore, a secondary aim of the current study was to examine the effects of sleep stage on EE during baseline and recovery sleep. We hypothesized that EE would vary between sleep stages and that EE would be lowest during SWS on the recovery night. As an exploratory aim, we examined the effects of sleep and sleep deprivation on substrate utilization.
Methods
Ethical approval
Study procedures were approved by the Scientific Advisory and Review Committee of the Colorado Clinical and Translational Sciences Institute, the Colorado Multiple Institutional Review Board, and by the Investigational Review Board at the University of Colorado Boulder. Written informed consent was obtained from subjects. The protocol was in accordance with the latest revision of the Declaration of Helsinki.
Subject characteristics
We studied seven subjects (5 males, 2 females) aged 22.4 ± 4.8 years (mean ± s.d.) with a BMI of 22.9 ± 2.4 kg m−2 (mean ± s.d.) and per cent body fat of 27.2 ± 7.2% (mean ± s.d.) as determined by dual-energy X-ray absorptiometry (DEXA; DXA, Model DPX-IQ Lunar Radiation Corporation, Madison, WI, USA). Subjects were determined healthy by physicians at the Clinical and Translational Research Centre (CTRC) based on physical exam, blood chemistries, 12-lead clinical electrocardiography, medical and psychiatric history. Furthermore, detailed health and sleep and circadian rhythm disorder history status was assessed at the Sleep and Chronobiology Laboratory and a polysomnographic (PSG) sleep disorder screening night verified subjects were free of sleep disorders. Exclusion criteria were any current or chronic medical or psychiatric conditions; shift work or dwelling below the Denver altitude (1600 m) in the year prior; travel across more than one time zone in the 3 weeks prior to the laboratory procedures; BMI outside the normal range of 18.5 to 24.9; recent self-reported weight loss; or physically active lifestyle, defined as greater than 1 h of structured exercise per week. Physically inactive subjects were studied to control for the effects of detraining during the sedentary laboratory procedures on energy expenditure. Furthermore, exercise was proscribed 2 days prior to the laboratory protocol to control for short-term effects of exercise on energy expenditure.
Subjects were instructed to refrain from consuming caffeine and alcohol for 3 days prior to the laboratory protocol to control for withdrawal and acute effects of these drugs on our primary outcome measures. Subjects self-reported they were medication free and urine toxicology verified subjects were drug free upon admission to the study. Female subjects were free of oral contraceptives and were studied during the week of menses (early follicular phase), or the first week post-menses during the follicular phase of the menstrual cycle to control for differences in sleep (Ito et al. 1993), thermoregulatory physiology (Wright & Badia, 1999), and energy expenditure that may be related to changes in reproductive hormones (Davidsen et al. 2007).
Pre-study control – ambulatory wakefulness, sleep recordings and energy intake
One week prior to the laboratory protocol, subjects were instructed to maintain a consistent ∼8 h per night sleep schedule. Compliance with this schedule was verified by call-in times to a time-stamped voice recorder of when subjects went to bed and awakened from sleep, daily sleep diaries and wrist actigraphy recordings (Actiwatch-L, Phillips Respironics, Bend, OR, USA). These ambulatory recording procedures ensured that subjects were not sleep deprived prior to the laboratory visit. Three days prior to the laboratory visit, energy intake was controlled by providing subjects with an isocaloric diet designed by the CTRC nutritionist.
Experimental procedures
Subjects were admitted to a research suite on the CTRC ∼6 h prior to their habitual bedtime (Fig. 1). Protocol events such as meals, bathroom breaks, bed and wake times and free time were scheduled according to the subjects’ habitual bedtime as determined by the sleep–wakefulness times during the week prior to entry into the laboratory (e.g. bedtime was assigned a relative clock hour of 24.00 h although actual bedtimes were determined by each subject's habitual sleep–wakefulness schedule). This procedure permitted subjects to sleep at their habitual circadian phase. A modified constant routine (Duffy & Wright, 2005) was employed to control for the influence of environmental and behavioural factors on our primary outcome measures. Specifically, during scheduled wakefulness, subjects were studied in a semi-recumbent posture with the head of the bed raised to ∼35 deg, ambient temperature was maintained in the thermoneutral range (22.3–22.9; 22.5 ± 0.02°C (range; mean ± s.d.)), and artificial lighting was maintained at dim levels (∼1.5 lux in the angle of gaze, <3 lux ambient; <8 lux maximum; IL-1400 photometer, International Light, Newburyport, MA, USA) to control for acute effects of light on temperature and endocrine physiology (Czeisler & Wright, 1999). Wakefulness and compliance with constant routine procedures were verified via continuous monitoring by research staff. If a subject showed signs of sleepiness, the subject was called by name and asked to change their activity (e.g. stop watching a movie or reading and engage in conversation). When not directly engaging subjects, continuous monitoring of EEG activity by staff ensured wakefulness.
Figure 1. Study protocol.
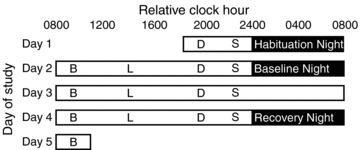
Black boxes represent scheduled sleep opportunities and white boxes represent scheduled wakefulness. Breakfast, lunch, dinner and a snack are denoted by B, L, D and S, respectively. Relative clock hour is on the abscissa and day of study is on the ordinate. Day 1 consisted of a habituation and sleep disorders screening night. Day 2 (baseline) consisted of 16 h of wakefulness and 8 h baseline sleep opportunity. Day 3 (sleep deprivation) consisted of 24 h of sleep deprivation. Day 4 (recovery) consisted of the remaining hours awake 25–40 h of sleep deprivation and 8 h recovery sleep. Energy expenditure was measured during days 2, 3 and 4.
Day 1 of the laboratory visit included an 8 h sleep opportunity to habituate subjects to the physiological recordings as well as to serve as a sleep disorders screen (Fig. 1). Day 2 served as baseline – scheduled 16 h wakefulness followed by an 8 h sleep opportunity. Days 3–4 consisted of 40 h total sleep deprivation followed by an 8 h recovery sleep episode (Sleep deprivation – 1 to 24 h awake; Recovery – 25 to 40 h awake and 8 h recovery sleep).
Energy expenditure and substrate utilization assessment
Energy expenditure and respiratory quotient (RQ) were determined in 1 min intervals from oxygen consumption and carbon dioxide production measured in a whole-room indirect calorimeter (Melanson et al. 2002). Gas concentrations were determined from the flow rate and the differences in CO2 and O2 concentrations between entering and exiting air by using Hartman and Braun (Frankfurt, Germany) CO2 (Uras 3G) and O2 (Magnos 4G) analysers. The analysers were calibrated daily using calibration gasses of known concentration. The accuracy and precision of the system is tested monthly using propane combustion tests. The expected volume of O2 and CO2 is determined based on expected production of 2.55 and 1.53 l of O2 and CO2, respectively, per gram of propane burned (Withers, 2001). The average O2 and CO2 recoveries during the course of this study were ≥95.0%. We have previously determined that the coefficient of variation in 24 h EE was 5.8% (unpublished data, ELM). Urine was collected throughout the duration of the calorimetry stay and analysed for total nitrogen concentration (Skogerboe et al. 1990) to determine 24 h protein oxidation (Livesey & Elia, 1988). Energy expenditure and substrate oxidation (fat and carbohydrate) were calculated from oxygen consumption and the non-protein RQ based on the equations of Jequier et al. (1987).
In-laboratory energy intake
Energy content of the diet was designed to meet individual daily energy requirements as determined by DEXA measurements of fat-free mass and an activity factor (pre-study, 1.5; in-laboratory, 1.2). Dietitians prepared isocaloric meals that contained macronutrient contents of 30% fat, 50% carbohydrate and 20% protein; 200 mEq Na+, 100 mEq K+; 2500 ml fluids and no caffeine. Meal content and timing was exactly the same for each day of laboratory study (Fig. 1) to control for thermic effects of food across days. A single subject did not finish one dinner during the 25–40 h of total sleep deprivation on day 4, otherwise subjects consumed the diet provided.
Polysomnography
Polysomnographic recordings were obtained with Siesta System digital sleep recorders (Compumedics USA Ltd, Charlotte, NC, USA). Electroencephalographic recordings were obtained from F3–A2, C3–A2, C4–A1 and O1–A2 according to the international 10–20 system. Sleep was scored in 30 s epochs according to standard guidelines from brain region C3–A2 or C4–A1 (Rechtschaffen & Kales, 1968). Sleep onset was defined as the first three continuous 30 s epochs of sleep (Wright et al. 1995).
Data analysis
Energy expenditure, substrate utilization and RQ were averaged for each 24 h day: Baseline (hours awake 1–16 and 8 h scheduled sleep), Sleep deprivation (hours awake 1–24) and Recovery (hours awake 25–40 and 8 h scheduled recovery sleep). Energy expenditure was also averaged for each scheduled wakefulness and sleep episode; and for each hour across the day. Data were aligned to habitual wake time, thus controlling for influence of circadian phase on EE within each individual (Spengler et al. 2000). Energy expenditure data during scheduled sleep episodes were also examined by wakefulness–sleep stage. Wakefulness–sleep stage and EE data were offset by 2 min to account for the lag time of response of the whole-room indirect calorimeter relative to the EEG recordings (Fontvieille et al. 1994). Average EE was calculated for the following stages: wakefulness prior to sleep onset (WPSO), wakefulness after sleep onset (WASO), stage 1 sleep, stage 2 sleep, slow-wave sleep (SWS; stages 3 and 4 sleep combined), and rapid eye movement sleep (REM). Individual differences in sleep stage duration, as well as the fact that sleep is scored in 30 s epochs and EE is estimated in 60 s epochs, required that we bin EE sleep stage data as follows: EE that corresponded to Stage 2 and SWS were averaged for episodes of ≥15 continuous minutes of the specific sleep stage, with the exception of one subject who had a maximum of 10.5 min of continuous SWS; EE that corresponded to REM sleep were averaged for episodes of ≥9.5 continuous minutes of REM, with the exception of one subject who had a maximum of 5 min of continuous REM. Stage 1 sleep, WPSO and WASO are short-duration transitional stages, therefore the average EE for episodes of ≥1 min of stage 1 sleep, and the average EE for episodes ≥30 s of WPSO and WASO were calculated. Unavoidably, WPSO and WASO episodes contained some sleep.
Statistical analysis
Non-parametric Wilcoxon matched pairs t tests were used to test for differences in total daily EE across days, scheduled wakefulness and sleep EE, and for 24 h RQ and substrate utilization. Repeated-measure ANOVAs were used to test for differences between average hourly EE and day of study (time of day × day interaction). Differences in sleep stage duration by sleep episode (scheduled baseline versus recovery sleep episode) were determined using t tests. Repeated-measure ANOVAs were also used to determine differences in EE between stages of sleep and wakefulness and between baseline and recovery sleep episodes (sleep–wakefulness stage × sleep episode interaction). For repeated-measure ANOVA analyses, Huynh–Feldt correction factors were used to correct for violations of homogeneity of variance and modified Bonferonni corrections were used to correct for the number of planned comparisons (Keppel, 1991).
Results
Effects of sleep, sleep deprivation and recovery sleep on energy expenditure
Figure 2 shows average hourly EE (kJ min−1) for each day of study. Energy expenditure was significantly higher during scheduled wakefulness versus sleep during the habitual nighttime (relative clock hours 24.00 to 08.00 h), and EE was higher following each scheduled meal during the habitual daytime (day × time of day interaction F = (40, 240) 10.68; P < 0.0001). Twenty-four hour EE (kJ day−1; Fig. 3A) was significantly higher during sleep deprivation compared to baseline and recovery days. On average, 24 h EE was ∼562 ± 8.61 kJ (∼134 ± 2.06 kcals) or ∼7% higher for sleep deprivation compared to baseline and ∼955 ± 97 kJ (∼228 ± 23 kcals) or ∼12% higher for sleep deprivation compared to recovery days (Fig. 3A). Furthermore, 24 h EE was on average ∼403 ± 106 kJ (∼96 ± 25 kcals) or ∼5% lower during recovery versus baseline days (Fig. 3A).
Figure 2. Energy expenditure for hourly averages for the three 24 h days.
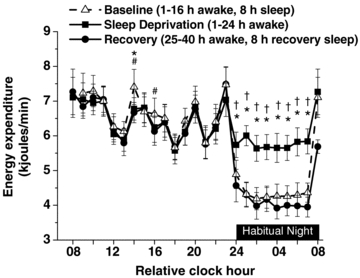
Data are plotted for relative clock hours with subjects’ habitual wake time arbitrarily assigned a value of 08.00 h. The black bar represents the habitual night when sleep was scheduled on baseline and recovery nights and during which sleep deprivation occurred. Relative clock hours 08.00 and 24.00 h represent habitual wake and bedtimes, respectively. Relative clock hour 24.00 h includes data binned from 24.00 to 00.59 h and thus is the first hour of the scheduled sleep episode. Relative clock hour 08.00 h consists of data binned from 08.00–08.59 h, and thus is the first hour of the scheduled wake episode. Peaks of EE during the habitual daytime are associated with the thermic effects of food. * represent significant differences (P < 0.05) between baseline and sleep deprivation (1–24 h awake). # represents significant differences (P < 0.05) between baseline and recovery (25–40 h awake/8 h recovery sleep). † represents significant differences (P < 0.05) between sleep deprivation (1–24 h awake) and recovery (25–40 h awake/8 h recovery sleep). Error bars represent s.e.m.
Figure 3. Total daily energy expenditure and energy expenditure associated with habitual wake and sleep episodes.
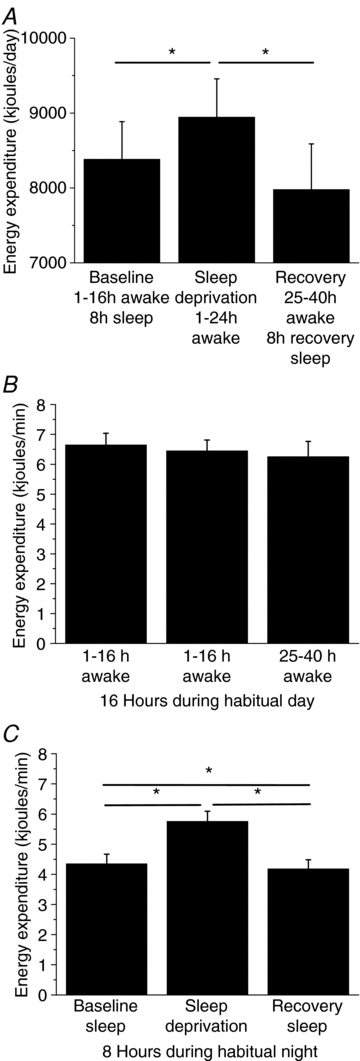
A, total daily EE in kJ day−1 for each day of the study. Energy expenditure in kJ min−1 during the 16 h habitual wake episode (B) and the 8 h habitual sleep episode (C). Lines represent significant differences between data at endpoints of the line. *P < 0.05. Error bars represent s.e.m.
Figure 3B shows that the average 16 h EE (kJ min−1) during the habitual daytime was similar regardless of sleep deprivation, whereas the average 8 h nighttime EE was significantly higher during sleep deprivation compared to baseline and recovery sleep episodes (Fig. 3C). Figure 3C also shows that the average 8 h nighttime EE was significantly lower during recovery versus baseline sleep. Specifically, 8 h nighttime EE was on average ∼673 ± 10.4 kJ (∼161 ± kcals) or ∼32% higher during nighttime sleep deprivation and on average ∼84 ± 3.1 kJ (∼28 ± 1.9 kcals) or ∼4% lower during nighttime recovery sleep when compared to the scheduled baseline sleep episode. Additionally, energy expenditure increased above baseline sleep levels in all subjects during the sleep deprivation night and decreased below baseline sleep levels in all but one subject during the recovery sleep night (Supplementary online material Fig. S1). There was no significant correlation between the magnitudes of change from baseline between sleep deprivation and recovery nights (r = −0.02, P = 0.97).
No statistical differences for average RQ and protein, fat and carbohydrate oxidation were observed between 24 h days (Supplementary online material Table S1).
Sleep–wakefulness stage and energy expenditure
Table 1 shows that sleep onset latency was significantly shorter and per cent of Stage 1, per cent time awake, and the number of arousals were significantly lower, whereas per cent SWS was significantly higher during the recovery compared to baseline sleep episode. The per cent of stage REM, stage 2 and duration of arousals were not significantly different between baseline and recovery sleep episodes. We also found that arousals were more likely to occur from REM and stage 2 sleep on baseline and recovery nights (Supplementary online material Fig. S2).
Table 1.
Polysomnographically (PSG) recorded sleep
| Measure | Baseline sleep episode | Recovery sleep episode |
|---|---|---|
| % of recording time | ||
| Stage 1 | 3.4 ± 0.8 | 1.7 ± 0.4* |
| Stage 2 | 54.4 ± 1.6 | 49.9 ± 3.3 |
| SWS | 18.5 ± 1.3 | 29.6 ± 2.6* |
| REM sleep | 18.7 ± 1.4 | 17.6 ± 1.9 |
| Wakefulness | 4.7 ± 1.0 | 2.0 ± 0.2* |
| Average number of arousals | 14.0 ± 1.7 | 10.6 ± 2.2* |
| Average duration of arousals (min) | 0.69 ± 0.1 | 0.67 ± 0.1 |
| Sleep onset latency (min) | 13.4 ± 3.9 | 3.5 ± 0.8* |
Sleep architecture (mean ± s.e.m) in per cent of the recording episode for sleep stages (n = 5), number and duration of arousals (n = 5) and sleep onset latency (n = 7) for the baseline and recovery nights. Wakefulness is the sum of WPSO and WASO. The sleep architecture analysis included 5 subjects because of PSG recording problems for 2 subjects. Note that percentages are rounded. *Significant dependent t test (P < 0.05) between sleep episodes.
Figure 4 shows the EE associated with sleep and wakefulness stages during baseline and recovery sleep episodes. A significant main effect of stage (F = (5, 25) 11.16; P < 0.00001) and planned comparisons revealed that average EE was significantly higher for WPSO compared to any sleep stage and to WASO during baseline and recovery sleep episodes. In addition, average EE was significantly higher for WASO compared to stage 2 sleep during baseline and recovery episodes. Furthermore, average EE was significantly higher for stage 1 compared to stage 2 sleep during the recovery episode. There was a non-significant trend of higher EE for WASO compared to SWS during the baseline episode (P = 0.053). Lastly, there were no significant differences in energy expenditure for a specific sleep stage between the baseline and recovery sleep episodes for sleep stage.
Figure 4. Energy expenditure during wakefulness and sleep stages.
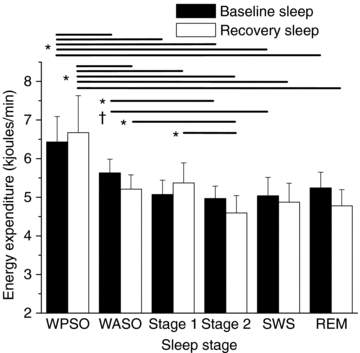
The EE associated with WPSO was significantly greater (P < 0.05) than any stage of sleep and WASO during the baseline (black) and recovery (white) nights. The EE associated with WASO was significantly different to stage 2 during the baseline and recovery nights. The EE associated with stage 1 was significantly different to stage 2 during the recovery night. The EE associated WASO had a non-significant trend for being different to SWS during the baseline night. Lines represent significant differences between stages at endpoints of the line. N = 6. Error bars represent s.e.m. *P < 0.05 between sleep stages within nights. †P = 0.053
Discussion
Sleep reduces EE and energy conservation is a hypothesized function of sleep. We quantified for the first time (1) the energy conserved by 8 h of sleep following 16 h of habitual wakefulness, and the energy conserved by 8 h of recovery sleep following one night of total sleep deprivation when sleep occurred at the habitual circadian phase; and (2) the metabolic cost of sleep deprivation assessed by 24 h EE in humans. We found that EE was higher during sleep deprivation compared to sleep and that EE was lower during an 8 h recovery sleep episode when compared to a habitual 8 h sleep episode. Sleep stage had little effect on EE with the exception of a small but significant difference in EE between stage 1 and stage 2 sleep during the recovery sleep episode. Wakefulness affected EE more than sleep stage with higher EE during WPSO than any sleep stage. Furthermore, EE during WASO was higher than EE during stage 2 sleep during baseline and recovery episodes.
Metabolic cost of sleep deprivation and the energy savings associated with sleep
Missing one night of sleep had a metabolic cost of ∼562 ± 8.6 kJ (∼134 ± 2.1 kcals) over 24 h, which equates to a ∼7% higher 24 h EE compared to a habitual day with an 8 h scheduled sleep episode. Thus, the metabolic cost of staying up all night, or conversely, the total daily energy savings associated with sleep, is similar to the energy content of two slices of bread or ∼225 ml of reduced fat 2% milk. Considered another way, the energetic cost of missing one night of sleep is similar to the energetic cost of a 68 kg adult walking ∼3 km at a moderate pace. This amount of energy savings by sleep in humans appears small, yet if one considers that the amount of positive energy storage needed to explain the obesity epidemic is ∼209 kJ day−1 (∼50 kcal day−1) (Hill et al. 2003), the ∼562 ± 8.6 kJ (∼134 kcals) energy savings we observed during sleep is physiological meaningful. Without sleep, humans would need a higher energy intake per day to meet total daily energy requirements. Our quantification of the 24 h metabolic cost of sleep deprivation was determined under controlled constant routine bed-rest conditions. It is likely that the metabolic cost of sleep deprivation in free-living individuals would have been even greater due to activity-associated energy demands (e.g. ambulation).
In rats, total daily EE was reported to be 50% higher during the first 24 h of total sleep deprivation compared to baseline (Everson et al. 1989b). In humans, we observed that total daily EE was ∼7% higher compared to baseline suggesting that there may be species differences in the metabolic response to sleep loss. Related, methods used for sleep deprivation were likely to be more metabolically challenging for prior studies of rats than in our study of humans (e.g. involuntary disk-over-water method and ad libitum food intake for rats vs. voluntary sleep deprivation in a thermoneutral bed-rest environment with an isocaloric diet in humans). Under disk-over-water conditions, total sleep deprivation is reported to increase ad libitum food intake in rats (Everson et al. 1989a) whereas it is unknown if total sleep deprivation increases ad libitum food intake in humans. However, energy intake has been reported to increase after a night of partial sleep deprivation (Brondel et al. 2010). It would be hypothesized that total sleep deprivation would increase food intake in humans as sleep deprivation has been reported to reduce levels of the satiety hormone leptin (Mullington et al. 2003) and to increase levels of the orexogenic hormone ghrelin and hunger ratings (Schmid et al. 2008). As noted, energy intake was controlled in the current study so that subjects consumed the energy needed to maintain energy balance under non-sleep-deprived conditions. We designed our study to examine the influence of sleep and sleep deprivation per se on EE. Thus, our control over activity and food intake does not permit us to make strong comments about how total daily EE and energy balance may change during uncontrolled conditions, something that should be examined in future studies.
Increases in 24 h EE during sleep deprivation were largely due to increases in EE during the habitual night. The observed decrease in EE during sleep supports a proposed function of sleep-energy conservation. Findings from prior studies indicated lower EE during sleep compared to wakefulness immediately prior to sleep (Kreider et al. 1958; Robin et al. 1958; Buskirk et al. 1960; White et al. 1985; Fraser et al. 1989; Bonnet et al. 1991). Methods used in prior studies did not allow distinction between decreases in EE due to time within the sleep episode and/or circadian phase, as no prior study compared EE during continuous wakefulness versus sleep across the habitual night. In the current study, we found that there was a 32% decrease in EE (∼673 kJ (∼161 kcal)) during sleep versus wakefulness during the 8 h habitual night. Thus, our findings demonstrate a relatively large sleep-dependent decrease in EE when controlling for time within the sleep episode and circadian phase within individuals.
Influence of recovery sleep on total daily EE and EE during the sleep episode
We found that 24 h EE was ∼403 ± 106 kJ (∼96 ± 25 kcals) or ∼5% lower during the recovery day compared to the baseline day. Thus, the 7% increase in 24 h EE on the sleep deprivation day was nearly offset by the energy saved during the recovery day, resulting in a net cost of 2% across the 48 h examined. It is likely that had we let subjects sleep ad libitum following sleep deprivation they would have slept longer (Dijk & Edgar, 1999), perhaps completely offsetting the entire metabolic cost of sleep deprivation. The latter suggests that under controlled feeding conditions needed to maintain weight, human metabolic physiology has the capacity to make adjustments to respond to the energetic cost of sleep deprivation and maintain energy balance across days. When we examined EE during the scheduled 8 h sleep episode, we found that EE was ∼84 kJ (∼20 kcals) or ∼4% lower during recovery than baseline sleep. Changes in many physiological processes, including reduced arousals, might contribute to the lower EE found during recovery than baseline sleep.
Energy expenditure and sleep architecture
We found that EE levels were higher for stage 1 sleep, a transitional stage between wakefulness and sleep, than for stage 2 during the recovery sleep episode and that EE levels were higher during WPSO than EE during all sleep stages during both baseline and recovery sleep episodes. We also found that EE levels were higher during WASO, brief arousals from sleep, than for stage 2 sleep during both baseline and recovery sleep episodes. Since sleep stage data are traditionally scored in 30 s epochs and EE data are averaged every minute, some of the EE associated with WASO included EE that was associated with sleep. Therefore, the EE associated with WASO in the current study might be an underestimate of the EE associated with brief awakenings. Regardless, we found the EE during WASO to be significantly higher than stage 2 sleep during the baseline and recovery nights. Overall, differences between sleep stages were relatively small whereas differences between wakefulness and sleep were more robust.
It is well established that sleep deprivation decreases the number of arousals and amount of WASO during recovery versus baseline sleep (De Gennaro et al. 2001; Curcio et al. 2003), which is consistent with the current findings, and thus, these changes might contribute to the lower EE we observed during recovery sleep. Since EE is higher during wakefulness than sleep, a reduction in sleep onset latency and the number of arousals during the scheduled sleep episode might be a primary mechanism of lower EE during recovery compared to baseline sleep in the current study. Additional support for the latter comes from findings of higher EE during tone fragmented sleep compared to a non-disturbed baseline night (Bonnet et al. 1991). Taken together, findings from Bonnet et al. (1991) and the current study suggest that factors that disrupt sleep such as environmental (e.g. noisy intensive care unit/bedroom), behavioural (e.g. consumption of caffeine) or pathophysiological (e.g. insomnia and sleep apnoea) might result in higher EE at night because of increased wakefulness and not a higher ‘sleeping’ energy expenditure per se. The latter hypotheses should be examined in future studies.
Findings from studies showing small differences in EE among sleep stages have generally indicated that EE was lowest during SWS compared to other stages of sleep (Brebbia & Altshuler, 1965; Fontvieille et al. 1994); however, as noted, significant differences in EE among sleep stages are not found in all studies (Webb & Hiestand, 1975; Haskell et al. 1981; White et al. 1985; Palca et al. 1986). It should be noted that prior findings of lower EE during slow-wave sleep compared to other sleep stages are limited by the fact that relatively short 5 min samples of EE were examined and that the EE levels were averaged across sleep stages that were ‘contaminated’ by other sleep and wakefulness stages in addition to the primary stage of interest (Brebbia & Altshuler, 1965; Fontvieille et al. 1994). Thus, it is possible that arousals from sleep could have influenced the EE results of such analyses. Indeed, we found that arousals from sleep were more likely to occur from stage 2 and REM sleep (Supplementary online material, Fig. S2), which could contribute to the reported higher EE during stage 2 and/or REM sleep compared to SWS in prior studies.
Energy conservation as a function of sleep in humans
As noted, our findings provide experimental support for the hypothesis that sleep conserves energy in humans and that the amount of energy conserved during sleep represents a physiologically meaningful amount of whole-body total daily energy expenditure. Why, however doesn't a night of sleep save more than ∼562 kJ (∼134 kcals) of total daily EE in humans? It is possible that the energy saved during sleep is the net result of decreases in the energetic cost of some basic life functions contributing to resting metabolic rate and a repartitioning of some of the energy saved for use in sleep-dependent physiological processes. For example, metabolically costly processes of respiration (Trinder et al. 1992), heart rate (Shinar et al. 2006), gut motility (Kumar et al. 1990) and muscle activity (Shinar et al. 2006) are reduced during sleep and some of the resulting energy saved may be redistributed to other metabolically costly processes that are proposed as sleep functions (e.g. sleep-dependent hormone synthesis and release (Sassin et al. 1969), increases in immune function (Everson, 1993; Palmblad et al. 1976; Bergmann et al. 1996), replenishment of cerebral glycogen stores (Benington & Heller, 1995) and synaptic plasticity (Cirelli et al. 2005)). Thus, based on our findings and findings from the existing literature, we propose that one of the functions of sleep in humans is to conserve energy and that some of the energy saved is redistributed to support other critical sleep-dependent physiological processes.
Acknowledgments
This project was supported by the Sleep Research Society Foundation, by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780, by NIH R01 HL08570, by the Undergraduate Research Opportunities Program and the Bioscience Undergraduate Research Skills and Training (BURST) of the Biological Sciences Initiative at the University of Colorado – Boulder. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The publications contents are the authors’ sole responsibility and do not necessarily represent official NIH views. We would like to thank the CTRC physicians, nurses, dieticians and technicians for study support.
Glossary
Abbreviations
- BMI
body mass index
- CTRC
Clinical Translational Research Centre
- DEXA
dual energy X-ray absorptiometry
- EE
energy expenditure
- RQ
respiratory quotient
- PSG
polysomnographic
- SWS
slow-wave sleep
- REM
rapid eye movement
- WASO
wakefulness after sleep onset
- WPSO
wakefulness prior to sleep onset
Author contributions
Experiments were performed at the Clinical and Translational Research Centre at the University of Colorado. All authors contributed to the following parts: (1) Conception and design of the experiment; (2) Collection, analysis and/or interpretation of data; (3) Drafting the article or revising it critically for important intellectual content. All authors approved the final version.
Author's present address
C. M. Jung: Department of Biology, University of Alaska Anchorage, Anchorage, AK 99508, USA
Supporting information
Supplementary Figure S1
Supplementary Figure S2
Supplementary Table S1
Supplementary Table S2
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Berger RJ, Phillips NH. Energy conservation and sleep. Behav Brain Res. 1995;69:65–73. doi: 10.1016/0166-4328(95)00002-b. [DOI] [PubMed] [Google Scholar]
- Bergmann BM, Gilliland MA, Feng PF, Russell DR, Shaw P, Wright M, Rechtschaffen A, Alverdy JC. Are physiological effects of sleep deprivation in the rat mediated by bacterial invasion? Sleep. 1996;19:554–562. doi: 10.1093/sleep/19.7.554. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Berry RB, Arand DL. Metabolism during normal, fragmented, and recovery sleep. J Appl Physiol. 1991;71:1112–1118. doi: 10.1152/jappl.1991.71.3.1112. [DOI] [PubMed] [Google Scholar]
- Brebbia DR, Altshuler KZ. Oxygen consumption rate and electroencephalographic stage of sleep. Science. 1965;150:1621–1623. doi: 10.1126/science.150.3703.1621. [DOI] [PubMed] [Google Scholar]
- Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–1559. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- Buskirk ER, Thompson RH, Moore R, Whedon GD. Human energy expenditure studies in the national institute of arthritis and metabolic diseases metabolic chamber. 1. Interaction of cold environment and specific dynamic effect. 2. Sleep. Am J Clin Nutr. 1960;8:602–613. [Google Scholar]
- Cirelli C, Huber R, Gopalakrishnan A, Southard TL, Tononi G. Locus ceruleus control of slow-wave homeostasis. J Neurosci. 2005;25:4503–4511. doi: 10.1523/JNEUROSCI.4845-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio G, Ferrara M, Pellicciari MC, Cristiani R, De Gennaro L. Effect of total sleep deprivation on the landmarks of stage 2 sleep. Clin Neurophysiol. 2003;114:2279–2285. doi: 10.1016/s1388-2457(03)00276-1. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Wright KP., Jr . Influence of light on circadian rhythmicity in humans. In: Turek FW, Zee PC, editors. Regulation of Sleep and Circadian Rhythms. New York: Marcel Dekker, Inc.; 1999. pp. 149–180. [Google Scholar]
- Davidsen L, Vistisen B, Astrup A. Impact of the menstrual cycle on determinants of energy balance: a putative role in weight loss attempts. Int J Obesity. 2007;31:1777–1785. doi: 10.1038/sj.ijo.0803699. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Bertini M. EEG arousals in normal sleep: Variations induced by total and selective slow-wave sleep deprivation. Sleep. 2001;24:673–679. doi: 10.1093/sleep/24.6.673. [DOI] [PubMed] [Google Scholar]
- Dijk D-J, Edgar DM. Circadian and homeostatic control of wakefulness and sleep. In: Turek FW, Zee PC, editors. Regulation of Sleep and Wakefulness. New York: Marcel Dekker, Inc.; 1999. pp. 111–147. [Google Scholar]
- Duffy JF, Wright KP., Jr Entrainment of the human circadian system by light. J Biol Rhy. 2005;28:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- Everson CA. Sustained sleep deprivation impairs host defense. Am J Physiol Regul Integr Comp Physiol. 1993;265:R1148–R1154. doi: 10.1152/ajpregu.1993.265.5.R1148. [DOI] [PubMed] [Google Scholar]
- Everson CA, Bergmann BM, Rechtschaffen A. Sleep-deprivation in the rat. III. Total sleep-deprivation. Sleep. 1989a;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- Everson CA, Gilliland MA, Kushida CA, Pilcher JJ, Fang VS, Refetoff S, Bergmann BM, Rechtschaffen A. Sleep-deprivation in the rat. IX. Recovery. Sleep. 1989b;12:60–67. [PubMed] [Google Scholar]
- Fontvieille AM, Rising R, Spraul M, Larson DE, Ravussin E. Relationship between sleep stages and metabolic-rate in humans. Am J Physiol Endocrinol Metab. 1994;30:E732–E737. doi: 10.1152/ajpendo.1994.267.5.E732. [DOI] [PubMed] [Google Scholar]
- Fraser G, Trinder J, Colrain IM, Montgomery I. Effect of sleep and circadian cycle on sleep period energy expenditure. J Appl Physiol. 1989;66:830–836. doi: 10.1152/jappl.1989.66.2.830. [DOI] [PubMed] [Google Scholar]
- Haskell EH, Palca JW, Walker JM, Berger RJ, Heller HC. Metabolism and thermoregulation during stages of sleep in humans exposed to heat and cold. J Appl Physiol. 1981;51:948–954. doi: 10.1152/jappl.1981.51.4.948. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: Where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Ito M, Kohsaka M, Fukuda N, Honma K, Honma S, Katsuno Y, Honma H, Kawai I, Morita N, Miyamoto T. Effects of menstrual-cycle on plasma melatonin level and sleep characteristics. Jpn J Psychiatry Neurol. 1993;47:478–479. doi: 10.1111/j.1440-1819.1993.tb02157.x. [DOI] [PubMed] [Google Scholar]
- Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Neutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher's Handbook. 3rd edn. Upper Saddle River: Prentice Hall; 1991. [Google Scholar]
- Kreider MB, Buskirk ER, Bass DE. Oxygen consumption and body temperatures during the night. J Appl Physiol. 1958;12:361–366. doi: 10.1152/jappl.1958.12.3.361. [DOI] [PubMed] [Google Scholar]
- Kumar D, Idzikowski C, Wingate DL, Soffer EE, Thompson P, Siderfin C. Relationship between enteric migrating motor complex and the sleep cycle. Am J Physiol Gastrointest Liver Physiol. 1990;259:G983–G990. doi: 10.1152/ajpgi.1990.259.6.G983. [DOI] [PubMed] [Google Scholar]
- Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr. 1988;47:608–628. doi: 10.1093/ajcn/47.4.608. [DOI] [PubMed] [Google Scholar]
- Melanson EL, Sharp TA, Seagle HM, Horton TJ, Donahoo WT, Grunwals GK, Hamilton JT, Hill JO. Effect of exercise intensity in 24-h energy expenditure and nutrient oxidation. J Appl Physiol. 2002;92:1045–1052. doi: 10.1152/japplphysiol.00706.2001. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Chan JL, Van Dongen HP, Szuba MP, Samaras J, Price NJ, Meier-Ewert HK, Dinges DF, Mantzoros CS. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–854. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- Palca JW, Walker JM, Berger RJ. Thermoregulation, metabolism, and stages of sleep in cold-exposed men. J Appl Physiol. 1986;61:940–947. doi: 10.1152/jappl.1986.61.3.940. [DOI] [PubMed] [Google Scholar]
- Palmblad J, Cantell K, Strander H, Froberg J, Karlsson CG, Levi L, Granstrom M, Unger P. Stressor exposure and immunological response in man – interferon-producing capacity and phagocytosis. J Psychosom Res. 1976;20:193–199. doi: 10.1016/0022-3999(76)90020-9. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, DC: U.S. Government Printing Office; 1968. [Google Scholar]
- Revell TK, Dunbar SG. The energetic savings of sleep versus temperature in the Desert Iguana (Dipsosaurus dorsalis) at three ecologically relevant temperatures. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:393–398. doi: 10.1016/j.cbpa.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Robin ED, Whaley RD, Crump CH, Travis DM. Alveolar gas tensions, pulmonary ventilation and blood pH during physiologic sleep in normal subjects. J Clin Invest. 1958;37:981–989. doi: 10.1172/JCI103694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassin JF, Parker DC, Mace JW, Gotlin RW, Johnson LC, Rossman LG. Human growth hormone release: relation to slow-wave sleep and sleep-walking cycles. Science. 1969;165:513–515. doi: 10.1126/science.165.3892.513. [DOI] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Jauchchara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17:331–334. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Shinar Z, Akselrod S, Dagan Y, Baharav A. Autonomic changes during wake-sleep transition: a heart rate variability based approach. Auton Neurosci. 2006;130:17–27. doi: 10.1016/j.autneu.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Skogerboe KJ, Labbe RF, Rettmer RL, Sundquist JP, Gargett AM. Chemiluminescent measurement of total urinary nitrogen for accurate calculation of nitrogen balance. Clin Chem. 1990;36:752–755. [PubMed] [Google Scholar]
- Spengler CM, Czeisler CA, Shea SA. An endogenous circadian rhythm of respiratory control in humans. J Physiol. 2000;526:683–694. doi: 10.1111/j.1469-7793.2000.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutain PL, Toutain C, Webster AJ, McDonald JD. Sleep and activity, age and fatness, and the energy expenditure of confined sheep. Br J Nutr. 1977;38:445–454. doi: 10.1079/bjn19770109. [DOI] [PubMed] [Google Scholar]
- Trinder J, Whitworth F, Kay A, Wilkin P. Respiratory instability during sleep onset. J Appl Physiol. 1992;73:2462–2469. doi: 10.1152/jappl.1992.73.6.2462. [DOI] [PubMed] [Google Scholar]
- Webb P, Hiestand M. Sleep metabolism and age. J Appl Physiol. 1975;38:257–262. doi: 10.1152/jappl.1975.38.2.257. [DOI] [PubMed] [Google Scholar]
- White DP, Weil JV, Zwillich CW. Metabolic rate and breathing during sleep. J Appl Physiol. 1985;59:384–391. doi: 10.1152/jappl.1985.59.2.384. [DOI] [PubMed] [Google Scholar]
- Wiersma P, Salomons HM, Verhulst S. Metabolic adjustments to increasing foraging costs of starlings in a closed economy. J Exp Biol. 2005;208:4099–4108. doi: 10.1242/jeb.01855. [DOI] [PubMed] [Google Scholar]
- Wright KP, Jr, Badia P. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res. 1999;103:185–194. doi: 10.1016/s0166-4328(99)00042-x. [DOI] [PubMed] [Google Scholar]
- Wright KP, Jr, Badia P, Wauquier A. Topographical and temporal patterns of brain activity during the transition from wakefulness to sleep. Sleep. 1995;18:880–889. doi: 10.1093/sleep/18.10.880. [DOI] [PubMed] [Google Scholar]
- Withers PC. Design, calibration, and calculation for flow-through respirometry systems. Aus J Zool. 2001;49:445–461. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



