Abstract
Study Objectives:
To investigate the relation between sleep duration and energy consumption in an adolescent cohort.
Design:
Cross-sectional.
Setting:
Free-living environment.
Participants:
Two hundred forty adolescents (mean age 17.7 ± 0.4 years).
Measurements and Results:
Daily 24-hour food-recall questionnaires and wrist-actigraphy measurements of sleep duration were employed to test the hypothesis that shorter weekday sleep duration (< 8 h) is associated with altered nutrient intake. Nutrition parameters included total calories, calories from meals and snacks, and proportions of caloric intake from fat and carbohydrates. Compared with adolescents sleeping 8 or more hours on average on weekdays, those sleeping less than 8 hours consumed a higher proportion of calories from fats (35.9% ± 6.7% vs 33.2% ± 6.9%; mean ± SD; P = 0.004) and a lower proportion of calories from carbohydrates (49.6% ± 8.2% vs 53.3% ± 8.3%; P = 0.001). After adjusting for potential confounders, shorter sleep duration was significantly associated with an average daily increase of calories consumed from fat of 2.2 percentage points and an average daily decrease in percentage of calories from carbohydrates of 3.0 percentage points. In unadjusted analyses, shorter sleep duration was also associated with a 2.1-fold increased odds (95% confidence interval: 1.03, 4.44) of daily consuming 475 or more kcal from snacks.
Conclusion:
Quantitative measures of macronutrient intake in adolescents were associated with objectively measured sleep duration. Short sleep duration may increase obesity risk by causing small changes in eating patterns that cumulatively alter energy balance.
Citation:
Weiss A; Xu F; Storfer-Isser A; Thomas A; Ievers-Landis CE; Redline S. The association of sleep duration with adolescents' fat and carbohydrate consumption. SLEEP 2010;33(9):1201-1209.
Keywords: Sleep duration, diet, obesity, adolescents, 24-hour food recall
OVER THE PAST 40 YEARS, INSUFFICIENT SLEEP DURATION AMONG ADOLESCENTS HAS MARKEDLY INCREASED, WHEREBY TODAY ONLY ABOUT 33% OF teens are getting the recommended 9 hours of sleep.1 Concurrent with this rise in short sleep duration is the epidemic of obesity.2 Obesity in children and adolescents is a growing health concern because of its adverse impact on metabolism, blood pressure, respiratory disease, and quality of life,3 as well as its association with adult obesity and chronic illnesses, including cardiovascular diseases, cancer, musculoskeletal disease, and gastrointestinal disease.4,5,6
A number of studies have suggested that there is a link between sleep loss and weight gain in adults.7–9 For children, this finding has been consistent across large samples studied in various countries10,11 and in prospective studies.12 Studies with older children or adolescents have found the relationship between short sleep duration and obesity for males but not for females.13–15 Physiologic studies have shown that sleep deprivation may influence weight through effects on appetite, physical activity, and/or thermoregulation.16,17 Experimental reductions in sleep duration have been hypothesized to alter metabolic rate or to affect the levels of appetite regulatory hormones, leptin and ghrelin.16–21 An association between insufficient sleep and increased caloric intake has been demonstrated in some,22 but not other,23 studies. Differences in the literature may relate to differences in experiment design. Understanding the association between insufficient sleep and obesity in the population has been limited by a paucity of research that has quantified both energy intake and sleep duration in individuals studied in their native environments. Thus, it is unclear whether the mechanisms that mediate sleep loss and energy balance are through appetitive pathways, changes in energy expenditure, or altered metabolism.
In this study, we examined the association between weekday sleep duration and energy consumption in adolescents aged 16 to 19 years studied in their natural environment. Using rigorously collected multiple-pass 24-hour food recalls and objectively measured sleep duration from wrist actigraphy, we tested the hypothesis that shorter sleep is associated with altered nutrient intake, including altered proportions of caloric intake from fats, carbohydrates, and snacks.
METHODS
Study Population
The study sample comprised adolescents participating in the late-adolescent examination of the Cleveland Children's Sleep and Health Study, an ongoing longitudinal cohort study designed to evaluate sleep measures and their health outcomes. As previously described,24 this birth cohort of children has been studied 3 times since 1998. For this examination, all cohort participants, now aged 16 to 19 years, were invited to participate. Although recruitment for this wave of data collection is ongoing, at the time of this report, data on macronutrient-intake and mean weekday sleep duration were available for 240 participants.
Study Protocol
Overnight polysomnography and physiologic and anthropometric assessments were performed using a standardized protocol in a dedicated clinical research facility. While at home during the week prior to the examination in the clinical research unit, participants wore a wrist actigraph and completed a daily sleep log for 5 to 7 consecutive 24-hour periods. Two complimentary 24-hour food recalls were administered, one conducted by phone during the week prior to the examination in the clinical research unit and the other in person at the clinical research unit. The protocol for this cross-sectional study was approved by the University Hospitals of Cleveland Institutional Review Board. Written consent and/or assent was obtained from the study participants for participants and their primary caregiver.
Measurements
Nutrition
To quantify total caloric and macronutrient intake as well as consumption of food from specific categories, dietary data were extracted from the 24-hour recall interviews conducted by trained staff using a multipass approach.25 This method is considered the “gold standard” for ascertaining a quantitative measure of food intake in epidemiologic nutrition studies. Currently used by the National Health and Nutrition Examination Surveys (NHANES), this standardized approach allows for numerous opportunities during the interview for subjects to recall their dietary intake. The interview includes collecting details about the food items and portion sizes, as well as details about the timing, location, meal type, and preparation of each item. The first pass of the collection phase allows for the participant to recall food items consumed over a 24-hour period, typically between midnight and midnight. During this pass, the subject is instructed to recall all food and beverage items consumed, with limited probing from the interviewer. The second pass involves probing by the interviewer to describe each food item with greater detail (i.e., percent of fat of milk consumed, addition of salt during the preparation, cut of meat). Food portions are verified using standard measuring utensils, rulers, and 2-dimensional food-portion visuals. The third pass is to review and ultimately confirm the dietary record with the subject. Validation studies have shown that data collected using this method agree between 10% and 15% with actual intake for children and adolescents.26,27 The nutrition data were analyzed using the Nutrition Data System for Research (NDS-R; developed by Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN), a comprehensive nutrient database including energy and macronutrients (NDS-R software versions 2007 and 2008).28
Energy consumption and macronutrient intake were calculated for each meal, during snacks, during 3 time periods throughout the day [early morning (05:00 to < 07:00), daytime (07:00 to < 21:00), and nighttime (21:00 to < 05:00)] and for each 24-hour period. Carbohydrate and fat intake were calculated as the percentage of total calories from carbohydrates and fats, respectively, averaged over the 2 recalls for 84.2% (n = 202) of individuals and from 1 available recall for 15.8% (n = 38) of individuals. Although the distributions of percentage of calories from carbohydrates and fats were approximately normal, the distribution of caloric intake from snacks revealed a 0-inflated, right-skewed distribution. Given this distribution, we chose to create a binary variable indicating high caloric intake from snacks using the top quintile of sample distribution (≥ 475 kcal).
Actigraphy
Sleep-wake estimation was made using wrist actigraphy (Octagonal Sleep Watch 2.01; AMI, Ambulatory Monitoring Inc., Ardsley, NY) analyzed using the Action-W software and the Time Above Threshold algorithm. Actigraphy was used, as opposed to the “gold-standard” polysomnography, in order to obtain estimates of sleep in free-living conditions. To enhance the reliability of estimated mean sleep duration, average weekday sleep duration was calculated for participants with at least 3 weekdays of actigraphy data. Though data on weekend sleep were available, they were not included in this analysis due to concerns that 1 to 2 days of weekend data would be poorly representative of a teen's usual weekend behavior that may change from week to week. Participants with a mean weekday sleep duration of less than 8 hours, which is approximately 1 sample SD (1.1 hours) less than the recommended amount of sleep for adolescents in this age group,29 were categorized as having shorter sleep.
Subject characteristics
Demographic data include age, sex, and race (African American versus other races). Preterm status was defined as a gestational age less than 37 weeks and was ascertained from birth records. Weight was obtained on a digital scale (Health-o-meter, Shelton, CT) and was calibrated daily using standard NST-certified weights. Height was obtained using a stationary stadiometer (Holtain Ltd, Pembrokeshire, UK), and was calibrated daily using a 600-mm calibration rod. Body mass index (BMI) was calculated as the ratio of weight to the square of height (kg/m2) and converted into age- and sex-adjusted percentiles.30 Obesity was defined as a BMI of 30 kg/m2 or greater or a BMI greater than the 95th percentile for age and sex. Socioeconomic status was determined as highest self-reported education of the parents and categorized as high school diploma or less, some college, or college degree.
Statistical Analyses
Subject characteristics, weekday sleep duration, and macronutrient intake were summarized using means and SD for normally distributed variables, medians and inter-quartile ranges (IQR) for markedly non-normally distributed variables, and counts and proportions for categorical variables. The primary exposure was defined as shorter mean weekday sleep duration (< 8 h; “shorter sleep”); mean weekday sleep duration as a continuous measure also was examined as a secondary exposure. The 3 outcome measures were the average percentage of daily calories consumed from fats, average percentage of daily calories consumed from carbohydrates, and high caloric intake from snacks (≥ 475 kcal; i.e., the highest quintile of calories from snacking). (Total daily energy consumption [kcal] also was examined; however, since 24-h recall interviews are considered to provide less reliable information on total energy consumption than proportionate macronutrient intake due to potential incomplete dietary reporting, it was not used as a primary outcome.) Linear regression was used to assess the association between sleep duration and percentage of daily calories from fats and carbohydrates. Two sets of multiple regression models were fit: (1) adjusted for subject characteristics that may confound the association between sleep duration and the outcome (age, sex, race, and parent education) and term status (a “design” variable used to adjust for the sampling design reflecting the construction of the cohort that was assembled to represent approximately equal proportions of children born prematurely and at term) and (2) adjusted for these prior subject characteristics as well as BMI, which may reflect adjustment for distal effects of sleep duration as well as for potential underreporting of caloric intake. Logistic regression was used to examine the relationship between sleep duration and the odds of high caloric intake from snacks. To avoid model overfitting due to small event size, the final adjusted logistic regression models did not include parent education as a covariate because this factor did not appreciably confound the association between sleep duration and high caloric intake from snacks. In exploratory analyses, the models were refitted stratifying by sex to assess possible effect modification by sex. Additional exploratory analyses were conducted to examine the relationship between sleep duration and time period when calories were consumed. Results are summarized using β coefficients and standard errors for linear regression models and adjusted odds ratios (aOR) and 95% confidence intervals (95% CI) for logistic-regression analyses. Statistical significance was set at 0.05 and no adjustments were made for multiple comparisons. Analyses were performed using SAS 9.1.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Subject Characteristics
The distributions of subject characteristics, including sleep duration and nutrition information, are shown in Table 1. Subjects were 17.7 years of age on average; approximately half were male (48%) and about one fifth of the sample was of African American race (21%). The mean height and weight for boys were 175.6 ± 6.5[± SD] cm and 77.3 ± 17.2 kg, respectively; on average, girls were 163.1 ± 7.2 cm tall and weighed 66.2 ± 17.4 kg. The median BMI for the sample was 23.1 kg/m2 (IQR 21.1, 27.1), and 18% were obese. Just over half of the participants had at least 1 parent with a college degree (51%). Mean weekday sleep duration ranged from 4.3 to 11.0 hours, with an average of 7.55 ± 1.14 hours. Only one-third (n = 81; 34%) slept at least 8 hours on weekdays on average. The median number of kilocalories consumed was 1917 (IQR 1573, 2403), and, on average, approximately half of those calories were from carbohydrates (50.8 ± 8.4) and one-third were from fat (35.0 ± 6.9). Three quarters of the adolescents reported eating snacks. As expected, the proportion of calories from carbohydrates and fats were strongly negatively correlated (r = −0.88). BMI was positively correlated with the proportion of calories consumed from fats (r = 0.17; P = 0.009) and negatively associated with the proportion of calories consumed from carbohydrates (r = −0.25; P < 0.001).
Table 1.
Sample characteristics for the total sample analyzed and stratified by mean weekday sleep duration
| Subject Characteristics | Total sample analyzeda (n = 240) | Sample according to mean weekday sleep durationb |
P Value | |
|---|---|---|---|---|
| < 8 h (n = 159) | ≥ 8 (n = 81) | |||
| Age, y | 17.7 ± 0.4 | 17.6 ± 0.4 | 17.7 ± 0.4 | 0.18 |
| Sex | ||||
| Male | 115 (47.9) | 88 (76.5) | 27 (23.5) | 0.001 |
| Female | 125 (52.1) | 71 (56.8) | 54 (43.2) | |
| Race | ||||
| African American | 50 (20.8) | 37 (74.0) | 13 (26.0) | 0.19 |
| Non-African American | 190 (79.2) | 122 (64.2) | 68 (35.8) | |
| Term status | ||||
| Preterm | 102 (42.5) | 66 (64.7) | 36 (35.3) | 0.66 |
| Full term | 138 (57.5) | 93 (67.4) | 45 (32.6) | |
| BMI, kg/m2 | 23.1 (21.1, 27.1) | 23.3 (21.4, 28.3) | 22.8 (20.6, 25.9) | 0.09 |
| Obesityc | ||||
| Obese | 44 (18.3) | 35 (79.6) | 9 (20.4) | 0.04 |
| Nonobese | 196 (81.7) | 124 (63.3) | 72 (36.7) | |
| Socioeconomic Status | ||||
| Highest parent education level | ||||
| ≤ High-school diploma | 38 (15.8) | 27 (71.1) | 11 (28.9) | |
| Some college | 79 (32.9) | 51 (64.6) | 28 (35.4) | 0.78 |
| College degree | 123 (51.3) | 81 (65.9) | 42 (34.1) | |
Data are presented as number (%) except age, which is mean ± SD, and body mass index (BMI), which is mean (interquartile range).
Column percentages shown;
Row percentages shown;
Obesity is defined as a BMI ≥ 95th percentile or BMI ≥ 30.
Bivariate analyses showed that adolescents who averaged less than 8 hours of sleep on weekdays did not significantly differ from those who slept at least 8 hours with respect to age, race, preterm status, or parent education (Table 1). However, the prevalence of shorter mean sleep duration was significantly higher for obese, compared with non-obese, adolescents (80% vs 63%, P = 0.04) as well as for boys, compared with girls (77% vs 57%, P = 0.001).
Sleep Duration and Average Daily Nutrient Intake
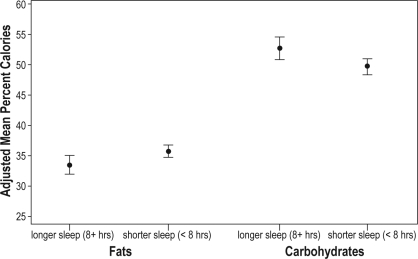
Unadjusted bivariate analyses showed that, on average, adolescents with shorter sleep duration (< 8 h) had a 2.7-percentage point increase in the proportion of energy from dietary fats and a 3.7-percentage point decrease in the proportion of energy from carbohydrates per day, compared with those who slept at least 8 hours on weekdays (fats: 35.9% ± 6.7% vs 33.2% ± 6.9% [mean ± SD], P = 0.004; carbohydrates: 49.6% ± 8.2% vs 53.3% ± 8.3%, P = 0.001) (Table 2). After adjusting for potential confounders, shorter sleep was significantly associated with an increase in the percentage of calories consumed from fats (average adjusted difference between those with shorter and longer sleep duration (2.2% ± 1.0% [mean ± SE], P = 0.02) and a decrease in the percentage of calories from carbohydrates (−3.0% ± 1.2%, P = 0.01) (Table 3 and Figure 1). Further adjusting for BMI, which may reflect “over” adjustment for distal effects of sleep duration, resulted in only a modest attenuation of these associations (Table 3). Secondary analyses stratified by sex and adjusted for participant characteristics suggest that the association between shorter sleep duration and increased consumption of calories from fats is significant among girls (3.3% ± 1.3%, P = 0.01) but not boys (0.9% ± 1.6%, P = 0.55). Similarly, the association between shorter sleep duration and decreased carbohydrates was significant in girls (−3.8% ± 1.5%, P = 0.01) but not boys (−2.2% ± 1.8%, P = 0.22).
Table 2.
Sample characteristics for the analytic sample and stratified by shorter mean weekday sleep duration
| Mean weekday sleep duration based on actigraphy | Total sample analyzeda (n = 240) | Sample according to mean weekday sleep durationb |
P Value | |
|---|---|---|---|---|
| < 8 h (n = 159) | ≥ 8 (n = 81) | |||
| Duration, h | 7.55 ± 1.14 | 6.91 ± 0.69 | 8.83 ± 0.67 | NA |
| Duration category, h | ||||
| < 6.5 | 38 (15.8) | 38 (100) | 0 (0) | NA |
| 6.5 to < 7 | 47 (19.6) | 47 (100) | 0 (0) | |
| 7 to < 8 | 74 (30.8) | 74 (100) | 0 (0) | |
| 8 to < 8.5 | 33 (13.8) | 0 (0) | 33 (100) | |
| ≥ 8.5 | 48 (20.0) | 0 (0) | 48 (100) | |
| Nutrition Measures | ||||
| Total energy, kcal | 1917 (1573, 2403) | 1968 (1618, 2461) | 1723 (1409, 2150) | 0.009 |
| Percentage of calories from | ||||
| Fat | 35.0 ± 6.9 | 35.9 ± 6.7 | 33.2 ± 6.9 | 0.004 |
| Carbohydrates | 50.8 ± 8.4 | 49.6 ± 8.2 | 53.3 ± 8.3 | 0.001 |
| Protein | 15.1 ± 3.6 | 15.4 ± 3.6 | 14.6 ± 3.7 | 0.13 |
| Calories consumed from snacks | 0.04 | |||
| Highest quintile | 51 (21.3) | 40 (78.4) | 11 (21.6) | |
| Other quintiles | 189 (78.7) | 119 (63.0) | 70 (37.0) | |
Data are shown as mean ± SD or number (%), except total energy, which is shown as is median (interquartile range).
Column percentages shown;
Row percentages shown;
The highest quintile included snacks comprising ≥ 475 kcal.
Table 3.
Association between shorter sleep duration and percentage of calories from fats and carbohydrates from multivariable linear regression models
| Subject Characteristics | Percentage of Calories from Fats |
Percentage of Calories from Carbohydrates |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||||||
| β | SE | P Value | β | SE | P Value | β | SE | P Value | β | SE | P Value | |
| Shorter Sleep | 2.21 | 0.95 | 0.02 | 2.01 | 0.95 | 0.04 | −3.00 | 1.16 | 0.01 | −2.52 | 1.15 | 0.03 |
| Age | −0.22 | 1.12 | 0.85 | −0.31 | 1.12 | 0.78 | 0.87 | 1.37 | 0.53 | 1.08 | 1.35 | 0.43 |
| Male sex | 1.24 | 0.91 | 0.17 | 1.26 | 0.90 | 0.16 | −1.83 | 1.11 | 0.10 | −1.88 | 1.09 | 0.08 |
| African American race | 2.88 | 1.14 | 0.01 | 2.64 | 1.14 | 0.02 | −2.56 | 1.39 | 0.07 | −2.00 | 1.38 | 0.15 |
| Preterm | 0.03 | 0.91 | 0.97 | −0.01 | 0.91 | 0.99 | 0.24 | 1.11 | 0.83 | 0.33 | 1.09 | 0.76 |
| Parent educationd | ||||||||||||
| ≤ HS diploma | 0.96 | 1.32 | 0.62 | 0.67 | 1.33 | 0.78 | −2.21 | 1.61 | 0.39 | −1.53 | 1.60 | 0.60 |
| Some college | 0.88 | 1.01 | 0.65 | 1.02 | −0.62 | 1.24 | −0.08 | 1.23 | ||||
| BMI | 0.13 | 0.08 | 0.11 | -0.30 | 0.10 | 0.002 | ||||||
aShorter sleep is defined as < 8 h; bCollege is the reference value.
Figure 1.
Mean and 95% confidence intervals for the percentage of calories from fats and carbohydrates, adjusted for age, sex, race, preterm status, and parent education in adolescents with average weekday sleep durations < 8 h and ≥ 8 h.
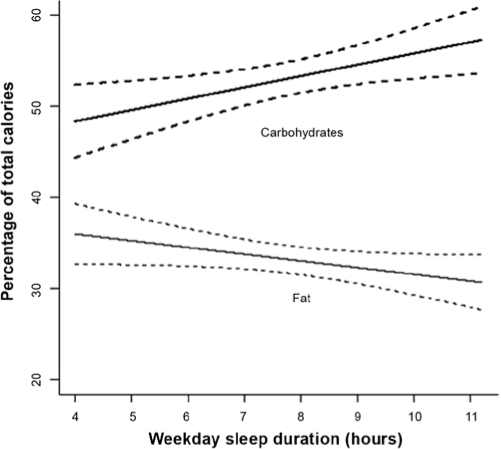
The results of the linear regression models defining the exposure as sleep duration continuously measured are summarized in Figure 2. The results are consistent with the findings when short sleep duration was modeled as a dichotomous exposure: decreased sleep duration was associated with increased consumption of calories from fats and decreased consumption of calories from carbohydrates. After adjusting for subject characteristics, the results show that, on average, each 1-hour decrease in sleep duration was associated with a 0.8-percentage point increase in consumption of calories from fats (0.8% ± 0.4%, P = 0.06) and a 1.2-percentage point decrease in consumption of calories from carbohydrates (−1.2% ± 0.5%, P = 0.01). The results of the sex-stratified analyses are also consistent with the primary analyses for percentage of calories from fats; the association between shorter sleep and increased consumption of calories from fats is significant among girls (−1.0% ± 0.6%, P = 0.06) but not among boys (−0.6% ± 0.6%, P = 0.31) after adjusting for subject characteristics. However, unlike the primary analysis, the magnitude of the associations between shorter sleep and percentage of calories from carbohydrates were similar for girls (1.3% ± 0.7%, P = 0.06) and boys (1.4% ± 0.7%, P = 0.05).
Figure 2.
Mean and 95% confidence interval of the association between mean weekday sleep duration and percentage of calories from fats and carbohydrates, adjusted for age, sex, race, preterm status, and parent education.
Sleep Duration and Nutrient Intake from Snacking
Shorter sleep duration was also associated with high caloric intake from snacks in unadjusted analyses; adolescents with a mean sleep duration of less than 8 hours had 2.1-fold increased odds of high caloric intake from snacks (OR = 2.14, 95% CI: 1.03, 4.44, P = 0.04). However, this association was attenuated and no longer statistically significant after adjusting for subject characteristics (aOR = 1.69, 95% CI: 0.79, 3.63, P = 0.18). Similar results were obtained when sleep duration was modeled as a continuous measure; after adjusting for subject characteristics, for each 1-hour increase in sleep duration, the odds of high caloric intake from snacks decreased by 21% on average (OR = 0.79, 95% CI: 0.59, 1.06, P = 0.12). In unadjusted sex-stratified analyses, the association between shorter sleep (< 8 h) and high caloric intake from snacks was stronger among girls (OR = 4.18, 95% CI: 1.14, 15.37, P = 0.03) than boys (OR = 1.00, 95% CI: 0.39, 2.56, P = 0.99). Due to small event size, these sex-stratified analyses were not adjusted for covariates.
Sleep Duration and Timing of Nutrient Intake
Exploratory analyses revealed that a significantly greater proportion of participants with shorter sleep duration (< 8 h) consumed food in the early morning period (05:00- < 07:00) compared with longer sleepers (25% vs 14%; P = 0.04). Among adolescents who ate during this time period, those with a shorter sleep duration consumed a larger proportion of their total daily calories (17.6% vs 10.9%, P = 0.04) and daily calories from carbohydrates (9.8% ± 6.5% vs 5.9% ± 4.7%, P = 0.04) between 05:00 and 07:00, compared with those who slept at least 8 hours on weekdays. In contrast, the proportion of adolescents reporting eating food at night (21:00-05:00) was similar for adolescents with shorter and longer sleep duration (41% vs 49%, P = 0.21), as were the proportions of calories from fats and carbohydrates and total daily calories consumed during this time period.
Sensitivity Analyses to Examine Potential Confounding Due to Underreporting
Exploratory and sensitivity analyses were used to examine under-reporting as a potential confounder of the results of this study. Although previous studies have shown that low energy-intake reporters (LER) tend to underestimate their fat intake,31 to the best of our knowledge, prior research has not examined the association between LER and sleep duration. In this study, LER could potentially bias the association between sleep duration and percentage of calories consumed from fats if participants with longer sleep durations were more likely to be LER. To examine the association between LER and sleep duration, we calculated the estimated basal metabolic rate (BMRest) for each participant,32 and adolescents with an energy intake-to-BMRest ratio less than 1.2 were classified as LER. The mean energy-intake:BMRest ratio in this study (1.22 ± 0.42) was somewhat higher than the mean reported in previous studies of adolescents,31,33–35 indicating less underreporting in this study. Exploratory bivariate comparisons of key subject characteristics by LER showed that the proportion of LER was not significantly different for participants with shorter and longer sleep durations (57.9% vs 51.9%, P = 0.38), and LER was not significantly associated with mean weekday sleep duration (as a continuous measure), age, sex, race, or preterm status (Table 4). Consistent with the literature, LER was positively associated with BMI and obesity.31
Table 4.
Key subject characteristics by LER status
| Not LER (n = 106) | LER (n = 134) | P Value | |
|---|---|---|---|
| Age, y | 17.7 ± 0.4 | 17.6 ± 0.4 | 0.56 |
| Sex | |||
| Male | 51 (44.4) | 64 (55.6) | 0.96 |
| Female | 55 (44.0) | 70 (56.0) | |
| Race | |||
| African American | 21 (42.0) | 29 (58.0) | 0.73 |
| Non-African American | 85 (44.7) | 105 (55.3) | |
| Term Status | |||
| Preterm | 45 (44.1) | 57 (55.9) | 0.99 |
| Full term | 61 (44.2) | 77 (55.8) | |
| BMI, kg/m2 | 22.0 (20.5, 24.6) | 24.7 (21.8, 29.3) | < 0.001 |
| Obesity | |||
| Obese | 11 (25.0) | 33 (75.0) | 0.005 |
| Nonobese | 95 (48.5) | 101 (51.5) | |
| Mean weekday sleep durationd | |||
| Duration, h | 7.65 ± 1.12 | 7.48 ± 1.15 | 0.27 |
| Short | 67 (42.1) | 92 (57.9) | 0.38 |
| Long | 39 (48.1) | 42 (51.9) |
Data are shown as number (%) except age, which is mean ± SD, and body mass index (BMI), which is median (interquartile range). LER refers to low energy-intake reporters.
aColumn percentages shown;
bRow percentages shown;
cObesity is defined as a BMI ≥ 95th percentile or BMI ≥ 30;
Sleep duration is defined as short (< 8 h) and long (≥ 8 h).
Sensitivity analyses were used to further address the possibility that LER underreported the proportion of calories consumed from fats, possibly confounding the results of this study. Based on previously reported differences in the percentage of calories consumed from fats by LER and non-LER participants,31 the proportion of calories consumed from fats by LER participants was modeled to increase from 1% to 5% in 1% increments, and the multivariable linear regression models with short sleep duration as the primary exposure were refitted. Additionally, these regression models were adjusted for characteristics associated with LER, including sex, race, and BMI.31 The results of the sensitivity analyses were similar to the original findings: shorter sleep duration was significantly associated with an increase in the proportion of calories consumed from fats (adjusted mean differences ranged from 2.03 ± 0.95 to 2.10 ± 0.97, P values < 0.05).
DISCUSSION
The key finding in this study was that macronutrient intake assessed using multiple-pass 24-hour food-recall interviews was associated with objectively measured sleep duration among adolescents studied in their natural environments. Specifically, shorter sleep duration was associated with a relative increase in caloric intake derived from fat and a decrease in caloric intake from carbohydrates. These findings persisted even after considering potential confounders, including sex, race, and parent education. These data extend the observations of Van Cauter and colleagues, who have shown that experimental sleep deprivation over several days in a laboratory setting results in increased levels of the appetite-stimulating hormone ghrelin, decreased levels of anorexinergic hormone leptin, and increased subjective ratings of hunger and appetite, with morning cravings for high-fat, high-carbohydrate foods.16,20,21,36,37 Our data are consistent with these experimental findings with regard to higher fat intake for adolescents who report shorter sleep durations and further suggest that levels of sleep deprivation commonly found in the population, especially in adolescents, are associated with unhealthy dietary habits.38–40 Furthermore, our data suggest that this association is significant among girls but not boys.
Nutrition intake was estimated using multiple-pass 24-hour dietary recalls and NDSR software, a research-quality, dietary, data-collection and nutrient-calculation tool specifically used in epidemiologic studies.25,41 Utilizing the multipass method, NDSR is highly accurate in assessing macronutrient intake.26,27 Weber et al., for example, have shown that children are able to correctly recall 75% of the foods they were observed consuming and correctly recall 83% of foods by food group.26 Still, because individuals may systematically underreport food intake, the NDSR multiple-pass approach is generally considered to provide more accurate data on proportionate intake of macronutrients rather than quantification of absolute nutrient and caloric intake. Therefore, our primary outcomes were proportion of calories derived from fats and carbohydrates, respectively. A higher proportion of calories consumed from fat may be interpreted as indicating greater energy intake. Although estimating total energy consumption with the use of this method has known limitations, our finding that shorter sleepers have a greater daily total caloric intake is consistent with this latter interpretation and suggests that shorter sleepers follow a dietary pattern that predisposes to weight gain. Increased fatty-food intake per se also may adversely impact health through the effects of dietary fat on lipogenesis42 and inflammatory cytokines,43 increasing the risk of developing chronic health disorders, such as high blood pressure and coronary artery disease.44
The link between short sleep and a pro-obesogenic dietary pattern has been documented in adults.16,21,22 Less data are available in children. In a large study of Finnish children ages 9 to 11 years old assessed by self-reported questionnaires, shorter weekday, but not weekend, sleep was associated with increased consumption of energy-rich foods.45 In a US study of adolescents, daytime sleep, but not nocturnal sleep, was associated with food cravings and hunger.46
Prior experimental research identified increased hunger for energy-rich foods, specifically, high carbohydrates, followed sleep restriction.21 Our results, showing that chronic sleep curtailment is associated with a proportionate increase in fat intake with a reciprocal proportionate decrease in carbohydrate intake, is not inconsistent with these findings. When macronutrients are expressed as proportions of total calories consumed, a reciprocal relationship between fat and carbohydrates is expected due to the relatively small daily proportion of calories associated with protein intake. An increased proportionate fat intake is consistent with an increase in absolute consumption of energy-rich foods. Diets that consist of a proportionate increase in carbohydrates are not necessarily unhealthy, since lower energy-dense diets may include carbohydrates derived from fruits, vegetables, beans, and other food sources. The health effects of carbohydrate intake may vary according to the types of carbohydrates consumed, as was shown by a longitudinal study of 572 healthy adults that found that BMI was not related to daily carbohydrate intake or to percentage of calories from carbohydrates. Instead, it was glycemic index, which is a measure of glycemic response associated with ingesting different types of carbohydrates, that was positively associated with BMI.47
The health impact of daily average increases of fat consumption by 2 to 3 percentage points is difficult to quantify. However, it is well known that positive energy balance that is small but persistent may cumulatively increase the risk of developing obesity.48 Our findings relating shorter sleep and changes in fat and carbohydrate consumption are remarkably similar to a recent report of Chinese adults who also underwent food-recall assessments but who had sleep duration estimated from questionnaires.49 Although the relative increase in fat consumption was small (2 to 3 percentage points), this dietary pattern, if chronically followed, could contribute to cumulative increases in energy consumption that would be expected to increase the risk of developing obesity. Long term, consistently over consuming energy can lead to an increased risk of developing obesity and over consuming calorie-dense foods that are high in fat that may contribute to overconsumption.50
Not only did macronutrient intake differ in shorter, as compared with longer, sleepers, but shorter sleepers also were more likely to consume high-energy snacks. Although these associations weakened after covariate adjustment, this finding is consistent with that of Nedeltcheva and colleagues who, in a laboratory study of 11 adults, reported that sleep curtailment to approximately 5.5 hours per night for 2 weeks led to increased caloric intake associated with snacking but no increase in calories from eating scheduled meals.22 Since, in their study, snacking was most common during the night and early morning hours, they concluded that sleep curtailment may increase energy consumption by providing greater opportunities to eat. Alternatively, given the well-recognized neural interconnections between food and sleep regulatory systems in the hypothalamus and the sensitivity of the amygdala to sleep deprivation, increased snacking and consumption of high-energy foods may reflect effects of sleep curtailment on stress responses and reward-seeking behaviors.18,51 As shown in animal studies52 and reports of shift workers,53 eating that occurs at times misaligned with circadian rhythms also may have particularly adverse metabolic consequences, leading to obesity and insulin resistance.54 Our exploratory analyses revealed that a greater proportion of short sleepers ate during the early morning hours. Given that most adolescents are “phase delayed,” it is possible that this eating pattern is misaligned with their circadian phase and could predispose them to developing adverse metabolic responses. However, additional research that includes measurement of circadian patterns concurrently with eating behaviors is needed to further understand the intersection among sleep duration, sleep timing, and eating behaviors.
Our data suggest that the association between short sleep and pro-obesogenic dietary habits may be stronger in girls than boys. The basis for sex differences is not presently understood. Studies examining sleep duration and weight in children have shown stronger associations in boys than girls.13 In a study of 9- to 11-year-old Finnish children, self-reported consumption of high energy-dense foods also was reported to be more strongly associated with short sleep in boys than in girls.45 In our sample of older adolescents, it is possible that eating behaviors in response to short sleep may have been influenced by socio-cultural or developmental influences. Considering the literature on sex differences in eating behaviors, our findings may be explained by an increased propensity of female adolescents, compared with males, for emotional eating.55 These observations suggest the importance of further research examining the links among sleep duration, stress, and eating behaviors, including emotional eating.55
Study strengths include objectively measured sleep duration in a community-based sample of adolescents studied in their natural environments. Macronutrient intake was also rigorously assessed using a multiple-pass 24-hour food-recall approach, which is considered to be the gold standard for research in nutrition epidemiology.25–27 There are limitations inherent to this diet-assessment technique, however, including the potential for underreporting of food intake.31 Still, for LER to confound the association between sleep duration and macronutrient intake, LER must be associated with sleep duration. Exploratory analyses revealed that LER was not significantly associated with mean weekday sleep duration or short sleep duration in the present study, and, thus, we do not have reason to believe that LER has significantly directed our findings. Ideally, 24-hour recalls should be administered over 3 periods to maximize reliability. The use of only 2 recalls may have reduced reliability, an effect that would be anticipated to bias the findings toward the null. Also, a small proportion of the sample answered only 1 recall, further reducing reliability. However, since neither sleep duration nor nutrient intake differed in these children, as compared with those completing 2 recalls, it is unlikely that this appreciably biased the results. Only 20% of this sample was of minority race, and further work is needed to assess the generalizability of these findings across population groups. Our findings show an association between sleep duration and macronutrient intake, but causation cannot be determined from a cross-sectional analysis.
In summary, chronic sleep deprivation and obesity have become increasingly prevalent in modern society, due to, among other factors, our over programmed lifestyles and frequent utilization of fast food, respectively. Emerging data indicate that these health conditions may be causally related through effects of sleep deprivation on thermoregulation56 and appetite-regulating hormones (leptin and ghrelin),19,37 autonomic function,57 and hypothalamic pituitary function.51 However, the relative influences of sleep deprivation on energy consumption, as compared with energy intake, are unclear. Our analyses suggest that short sleepers engage in pro-obesogenic eating behaviors, specifically, ingesting increased amounts of fatty foods, and also eat more in the early morning. These findings underscore the importance in targeting sleep behaviors for health promotion and obesity prevention and suggest a role for improving sleep duration as a component of weight management programs.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Redline has received the use of equipment from Respironics and has received research support from Dymedix Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by NIH grants: NIH HL07567, HL60957, UL1-RR024989, and 1U54CA116867.
Footnotes
A commentary on this article appears in this issue on page 1135.
REFERENCES
- 1.Bonnet MH, Arand DL. We are chronically sleep deprived. Sleep. 1995;18:908–11. doi: 10.1093/sleep/18.10.908. [DOI] [PubMed] [Google Scholar]
- 2.Patel SR, Redline S. Two Epidemics: Are we getting fatter as we sleep less? Sleep. 2004;27:602–3. [PubMed] [Google Scholar]
- 3.Gunturu SD, Ten S. Complications of obesity in childhood. Pediatr Ann. 2007;36:96–101. doi: 10.3928/0090-4481-20070201-08. [DOI] [PubMed] [Google Scholar]
- 4.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–6. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120:340–5. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 6.Cheung YB, Machin D, Karlberg J, Khoo KS. A longitudinal study of pediatric body mass index values predicted health in middle age. J Clin Epidemiol. 2004;57:1316–22. doi: 10.1016/j.jclinepi.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12:289–298. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Hasler G, Buysse D, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–6. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 9.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the ‘Quebec en Forme’ project. Int J Obes (Lond) 2006;30:1080–5. doi: 10.1038/sj.ijo.0803291. [DOI] [PubMed] [Google Scholar]
- 11.Sekine M, Yamagami T, Handa K, et al. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health Dev. 2002;28:163–70. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 12.Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr. 2004;145:20–5. doi: 10.1016/j.jpeds.2004.03.023. Erratum in: J Pediatr. 2004;145:424. [DOI] [PubMed] [Google Scholar]
- 13.Knutson KL. Sex differences in the association between sleep and body mass index in adolescents. J Pediatr. 2005;147:830–4. doi: 10.1016/j.jpeds.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Eisenmann JC, Ekkekakis P, Holmes M. Sleep duration and overweight among Australian children and adolescents. Acta Paediatr. 2006;95:956–63. doi: 10.1080/08035250600731965. [DOI] [PubMed] [Google Scholar]
- 15.Ievers-Landis CE, Storfer-Isser A, Rosen C, Johnson NL, Redline S. Relationship of sleep parameters, child psychological functioning, and parenting stress to obesity status among preadolescent children. J Dev Behav Pediatr. 2008;29:243–52. doi: 10.1097/DBP.0b013e31816d923d. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 17.Krauchi K, Deboer T. The interrelationship between sleep regulation and thermoregulation. Front Biosci. 2010;15:604–25. doi: 10.2741/3636. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel K, Leproult R, Van Cauter E. The impact of sleep debt on physiological rhythms. Rev Neurol (Paris) 2003;159(Suppl 11):6S11–20. [PubMed] [Google Scholar]
- 19.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–78. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel K, Leproult R, Tasali E, Penev P, Van Cauter E. Sleep curtailment results in decreased leptin levels and increased hunger and appetite. Sleep. 2003;26:A174. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 22.Nedeltcheva AV, Kilkus JM, et al. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmid SM, Hallschmid M, Jauch-Chara, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–82. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 24.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep Quality and Elevated Blood Pressure In Adolescents. Circulation. 2008;118:1034–40. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guenther PM, De Maio UJ, Ingwersen LA, Berlin MC. The multi-pass approach for 24-hr recalls in the continuing survey of food intakes by individuals. FASEB J. 1996;10:A198. [Google Scholar]
- 26.Weber JL, Lytle L, Gittelsohn J, et al. Validity of self-reported dietary intake at school meals by American Indian children: the pathways study. J Am Diet Assoc. 2004;104:746–52. doi: 10.1016/j.jada.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 27.Jonnalagadda SS, Mitchell DC, Smiciklas-Wright H, et al. Accuracy of energy intake data estimated by a multiple-pass, 24-hour dietary recall technique. J Am Diet Assoc. 2000;11:303–8. doi: 10.1016/s0002-8223(00)00095-x. [DOI] [PubMed] [Google Scholar]
- 28.Schukel SF. Maintaining a nutrient database in a changing market place: keeping pace with changing food products—a research perspective. J Food Comp Anal. 2001;14:315–22. [Google Scholar]
- 29.National Sleep Foundation. Adolescent Sleep Needs and Patterns 2000. www.sleepfoundation.org/publications/sleep_and_teens_report1.pdf.
- 30.Atlanta, GA: Center for Disease Control and Prevention; 2000. 2000 CDC Growth Charts: United States. [Google Scholar]
- 31.Livingstone MBE, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133:895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- 32.Henry CJ. Basal metabolic rate studies in humans: measurements and development of new equations. Public Health Nutr. 2005;8:1133–52. doi: 10.1079/phn2005801. [DOI] [PubMed] [Google Scholar]
- 33.Bandini LG, Schoeller DA, Cyr H, Dietz WH. Validity of reported energy intake in obese and nonobese adolescents. Am J Clin Nutr. 1990;52:421–5. doi: 10.1093/ajcn/52.3.421. [DOI] [PubMed] [Google Scholar]
- 34.Bratteby LE, Sandhagen B, Fan H, Enghardt H, Samuelson G. Total energy expenditure and physical activity as assessed by the doubly labeled water method in Swedish adolescents in whom energy intake was underestimated by 7-d diet records. Am J Clin Nutr. 1998;67:905–11. doi: 10.1093/ajcn/67.5.905. [DOI] [PubMed] [Google Scholar]
- 35.Livingstone MBE, Prentice AM, Coward WA, et al. Validation of estimates of energy intake by weighed dietary record and diet history in children and adolescents. Am J Clin Nutr. 1992;56:29–35. doi: 10.1093/ajcn/56.1.29. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 37.Taheri S, Lin L, Austin D, Young T, Mignot E. Short Sleep Duration is Associated with Reduced Leptin, Elevated Ghrelin, and Increased Body Mass Index. PLOS Med. 2004;1:210. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 39.Singh M, Drake CL, Roehrs T, Hudgel DW, Roth T. The association between obesity and short sleep duration: a population-based study. J Clin Sleep Med. 2005;1:357–63. [PubMed] [Google Scholar]
- 40.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schukel SF, Buzzard IM, Gebhardt SE. Procedures for estimating nutrient values for food composition databases. J Food Comp Anal. 1997;10:102–14. [Google Scholar]
- 42.Minihane AM. Nutrient gene interactions in lipid metabolism. Curr Opin Clin Nutr Metab Care. 2009;12:357–63. doi: 10.1097/MCO.0b013e32832c94a5. [DOI] [PubMed] [Google Scholar]
- 43.Ma X, Torbenson M, Hamad AR, Soloski MJ, Li Z. High-fat diet modulates non-CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin Exp Immunol. 2008;151:130–8. doi: 10.1111/j.1365-2249.2007.03530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu FB. Diet and lifestyle influences on risk of coronary heart disease. Curr Atheroscler Rep. 2009;11:257–63. doi: 10.1007/s11883-009-0040-8. [DOI] [PubMed] [Google Scholar]
- 45.Westerlund L, Ray C, Roos E. Associations between sleeping habits and food consumption patterns among 10-11-year old children in Finland. Br J Nutr. 2009;102:1531–7. doi: 10.1017/S0007114509990730. [DOI] [PubMed] [Google Scholar]
- 46.Landis AM, Parker KP, Dunbar SB. Sleep, hunger, satiety, food cravings, and caloric intake in adolescents. J Nurs Scholarsh. 2009;41:115–23. doi: 10.1111/j.1547-5069.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 47.Ma Y, Olendzki B, Chiriboga D, et al. Association between dietary carbohydrates and body weight. Am J Epidemiol. 2005;16:359–67. doi: 10.1093/aje/kwi051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocrinol Rev. 2006;27:750–61. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- 49.Shi Z, McEvoy M, Luu J, Attia J. Dietary fat and sleep duration in Chinese men and women. Int J Obes (Lond) 2008;32:1835–40. doi: 10.1038/ijo.2008.191. [DOI] [PubMed] [Google Scholar]
- 50.Bray GA, Popkin BM. Dietary fat intake does affect obesity'. Am J Clin Nutr. 1998;68:1157–73. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- 51.Van Cauter E, Holmback U, Knutson K, et al. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67(Suppl 1):2–9. doi: 10.1159/000097543. [DOI] [PubMed] [Google Scholar]
- 52.Ramsey KM, Marcheva B, Kohsaka A, Bass J. The clockwork of metabolism. Annu Rev Nutr. 2007;27:219–40. doi: 10.1146/annurev.nutr.27.061406.093546. [DOI] [PubMed] [Google Scholar]
- 53.Holmbäck U, Forslund A, Lowden A, et al. Endocrine responses to nocturnal eating—possible implications for night work. Eur J Nutr. 2003;42:75–83. doi: 10.1007/s00394-003-0386-6. [DOI] [PubMed] [Google Scholar]
- 54.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen-Rodriguez ST, Unger JB, Spruijt-Metz D. Psychological determinants of emotional eating in adolescence. Eating Disord. 2009;17:211–24. doi: 10.1080/10640260902848543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng PF, Shaw P, Bergmann BM, et al. Sleep deprivation in the rat: XX. Differences in wake and sleep temperatures during recovery. Sleep. 1995;18:797–804. doi: 10.1093/sleep/18.9.797. [DOI] [PubMed] [Google Scholar]
- 57.Sgoifo A, Buwalda B, Roos M, Costoli T, Merati G, Meerlo P. Effects of sleep deprivation on cardiac autonomic and pituitary-adrenocortical stress reactivity in rats. Psychoneuroendocrinology. 2006;31:197–208. doi: 10.1016/j.psyneuen.2005.06.009. [DOI] [PubMed] [Google Scholar]