Abstract
The ex vivo antiviral CD8+ repertoires of 34 human immunodeficiency virus (HIV)-seropositive patients with various CD4+ T-cell counts and virus loads were analyzed by gamma interferon enzyme-linked immunospot assay, using peptides derived from HIV type 1 and Epstein-Barr virus (EBV). Most patients recognized many HIV peptides, with markedly high frequencies, in association with all the HLA class I molecules tested. We found no correlation between the intensity of anti-HIV CD8+ responses and the CD4+ counts or virus load. In contrast, the polyclonality of anti-HIV CD8+ responses was positively correlated with the CD4+ counts. The anti-EBV responses were significantly less intense than the anti-HIV responses and were positively correlated with the CD4+ counts. Longitudinal follow-up of several patients revealed the remarkable stability of the anti-HIV and anti-EBV CD8+ responses in two patients with stable CD4+ counts, while both antiviral responses decreased in two patients with obvious progression toward disease. Last, highly active antiretroviral therapy induced marked decreases in the number of anti-HIV CD8+ T cells, while the anti-EBV responses increased. These findings emphasize the magnitude of the ex vivo HIV-specific CD8+ responses at all stages of HIV infection and suggest that the CD8+ hyperlymphocytosis commonly observed in HIV infection is driven mainly by virus replication, through intense, continuous activation of HIV-specific CD8+ T cells until ultimate progression toward disease. Nevertheless, highly polyclonal anti-HIV CD8+ responses may be associated with a better clinical status. Our data also suggest that a decrease of anti-EBV CD8+ responses may occur with depletion of CD4+ T cells, but this could be restored by highly active antiretroviral treatment.
Human immunodeficiency virus (HIV) infection results in intense activation of the immune system, especially of virus-specific cytotoxic T lymphocytes (CTL). These anti-HIV CTL are a major factor in the control of virus load, as especially well demonstrated by recent studies of CD8+ T-cell depletion in macaques (32, 41). Even though it is generally believed that anti-HIV cytotoxic responses are more efficient since they are broader and more intense, their characteristics involved in increased host resistance to progression toward AIDS remain to be clearly identified (16, 29). Indeed, the number of effector CTL (CTLe) detected ex vivo and their role in the evolution of HIV infection are still widely debated (2, 15, 18, 20, 37, 38, 42). Investigation of the exact relationship between the degree of activity of anti-HIV CTL and the rate of progression toward AIDS is hindered by several factors (29), especially the diversity of often conflicting techniques used, which sometimes address qualitatively different CD8+ T-cell subsets, and the lack of a rigorous quantitative assay. The limiting dilution assay (LDA) commonly used to quantify virus-specific CTL yields frequencies of anti-HIV CD8+ T cells that represent only a small fraction of the total number of CD8+ T cells in HIV-infected patients. In contrast, the percentage of lymphocytes bearing activation markers such as CD38 or HLA-DR and lacking CD28 is greatly increased in HIV infection, frequently reaching over 30% of total CD8+ T cells (7, 15). In fact, LDA has recently been shown to underestimate the in vivo frequency of anti-HIV CD8+ T cells compared with other newly developed quantitative assays (1, 26, 33, 34). Ex vivo measurements of gamma interferon (IFN-γ) secretion by single cells, using an enzyme-linked immunospot (ELISPOT) assay, appeared to be a particularly simple and sensitive method to quantify CD8+ T lymphocytes specific for a pathogen (26, 27, 34, 40). We used an IFN-γ ELISPOT assay to study ex vivo the reactivity of the CD8+ T cell of 34 HIV type 1 (HIV-1)-seropositive patients against many optimal HLA class I epitopes derived from the sequences of HIV-1 and Epstein-Barr virus (EBV). Three parameters were defined to describe the antiviral repertoire of the patients: (i) polyclonality (number of viral epitope peptides recognized), (ii) total intensity (sum of the numbers of antiviral CD8+ T cells per 106 peripheral blood mononuclear cells [PBMC] for all viral peptides recognized), and (iii) mean intensity (total intensity divided by polyclonality). We have used these data to compare the intensities of anti-HIV and anti-EBV CD8+ T-cell responses ex vivo, to determine whether some parameters of ex vivo anti-HIV or anti-EBV responses are correlated with the CD4+ T cell counts or virus load, to follow the evolution of anti-HIV and anti-EBV responses in several patients to determine whether the patterns of evolution of antiviral responses are linked to specific patterns of clinical evolution, and to assess the impact of highly active antiretroviral treatment (HAART) on the numbers of anti-HIV and anti-EBV CD8+ T cells.
MATERIALS AND METHODS
Subjects.
We studied retrospectively anti-HIV CD8+ T lymphocytes obtained from 34 HIV-1-infected patients and from 10 healthy HIV-seronegative volunteers who served as control subjects. Cohorts were established with the approval of the local ethics committee (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale de l’Hôpital Cochin), and all participants gave their written informed consent for the constitution of cell banks.
Cells.
PBMC isolated from freshly drawn heparinized venous blood were isolated by density gradient centrifugation (separation medium; Flow, Irvine, United Kingdom) and used after freezing and thawing. Subjects were serologically HLA typed by complement-mediated lymphocytotoxicity.
Peptides.
The HIV-1 and EBV epitopes used are listed in Table 1. The sequences of these epitopes are available online (19a, 32a). Peptides were synthesized by Neosystem (Strasbourg, France) and supplied by the Agence Nationale de la Recherche sur le SIDA. Lyophilized peptides were diluted to 1 mg/ml in water plus 10% dimethyl sulfoxide aliquoted, and stored at −20°C. They were used at a final concentration of 1 μg/ml (0.01% dimethyl sulfoxide in cell culture medium).
TABLE 1.
HIV-1 and EBV epitopic peptides used
| HLA | Virus | Peptide | HLA | Virus | Peptide | |
|---|---|---|---|---|---|---|
| A1 | HIV | Nef 121–128 | A31 | HIV | Env 775–785 | |
| HIV | Nef 184–191 | HIV | Gag 20–28 | |||
| A2 | EBV | LMP-2 426–434 | HIV | Gag 83–91 | ||
| HIV | Env 37–46 | HIV | Nef 84–92 | |||
| HIV | Env 121–129 | A32 | HIV | Env 424–432 | ||
| HIV | Gag 77–85 | HIV | Env 774–782 | |||
| HIV | Pol 200–208 | HIV | Pol 589–568 | |||
| HIV | Pol 476–484 | B7 | EBV | EBNA-3A 379–387 | ||
| HIV | Pol 588–596 | EBV | EBNA-3C 881–891 | |||
| HIV | Pol 683–692 | HIV | Env 303–312 | |||
| HIV | Nef 136–145 | HIV | Env 848–856 | |||
| A3 | HIV | Env 775–785 | HIV | Nef 68–77 | ||
| HIV | Gag 18–26 | HIV | Nef 128–137 | |||
| HIV | Gag 20–28 | B8 | EBV | EBNA-3 325–33 | ||
| HIV | Gag 266–275 | HIV | Env 591–598 | |||
| HIV | Pol 325–333 | HIV | Env 851–859 | |||
| HIV | Nef 73–82 | HIV | Gag 24–32 | |||
| A11 | EBV | EBNA-4 416–424 | HIV | Gag 259–267 | ||
| HIV | Gag 83–91 | HIV | Gag 329–337 | |||
| HIV | Gag 349–359 | HIV | Pol 185–193 | |||
| HIV | Pol 325–333 | HIV | Nef 90–97 | |||
| HIV | Pol 507–517 | B27 | HIV | Env 791–800 | ||
| HIV | Nef 73–82 | HIV | Gag 263–272 | |||
| HIV | Nef 84–92 | HIV | Nef 134–141 | |||
| A24 | HIV | Env 590–598 | B35 | EBV | EBNA-3 458–466 | |
| HIV | Pol 508–517 | HIV | Gag 254–262 | |||
| HIV | Nef 134–143 | HIV | Pol 342–350 | |||
| A25 | HIV | Nef 135–143 | HIV | Nef 68–77 | ||
| A30 | EBV | EBNA-3A 603–611 | HIV | Nef 74–81 | ||
| HIV | Gag 20–28 | HIV | Nef 135–143 | |||
| HIV | Nef 73–82 | B44 | EBV | EBNA-3C 163–171 | ||
| EBV | EBNA-6 290–299 | |||||
| HIV | Gag 178–186 |
ELISPOT assay.
The IFN-γ ELISPOT assay was adapted from that of Scheibenbogen et al. (40). Ninety-six-well nitrocellulose plates (Millipore, Bedford, Mass.) were coated with capture mouse anti-human IFN-γ monoclonal antibody (2 μg/ml; code 1598-00; Genzyme, Rüsselheim, Germany). PBMC, either freshly isolated or thawed and cultured overnight in complete medium, were plated in triplicate at serial dilutions (3 × 105 to 104 cells per well). Appropriate stimuli were then added, and the plates were incubated for 20 h at 37°C and 5% CO2. After the plates were washed, the cells were incubated with 100 μl of rabbit polyclonal anti-human IFN-γ antibody (code IP500; 1:250 dilution; Genzyme), then with an anti-rabbit immunoglobulin G-biotin conjugate (1:500 dilution; Boehringer, Mannheim, Germany), and finally with alkaline phosphatase-labeled extravidin (Sigma, St. Louis, Mo.). Spots were developed by adding chromogenic alkaline phosphatase conjugate substrate (BioRad, Hercules, Calif.). Colored spots were counted in a stereomicroscope. The signal was considered positive if the number of spots was significantly greater than that obtained with the negative controls at least at one of the cell concentrations used and if the number of spots obtained was proportional to the number of plated cells. Frequencies of IFN-γ spot-forming cells (SFC) were then calculated.
Positive controls consisted of six wells containing 300 to 1,000 cells with phorbol myristate acetate (50 ng/ml) and ionomycin (500 ng/ml). This strong mitogenic stimulus permitted verification that freezing and thawing various cell samples did not introduce artifactual differences in T-cell reactivities, thus constituting an indirect check of overall T-lymphocyte viability.
Negative controls consisted of HLA-matched and mismatched epitopic peptides derived from HIV or other viruses (including the HLA-A2-restricted peptide Tax 11-19, derived from the irrelevant human T-cell lymphotropic virus type 1). Negative controls never elicited a specific response compared with PBMC incubated in medium alone. The PBMC from the HIV-seronegative individuals secreted no IFN-γ in response to HIV peptides, but they did in response to some EBV or influenza virus peptides, depending on their HLA haplotype (data not shown). For some HIV-1-infected patients, we checked that all epitopes recognized in the IFN-γ ELISPOT assay were able to expand antipeptide cell lines. ELISPOT assays performed on purified T-cell subsets showed that only CD8+ T cells secreted IFN-γ in response to viral HLA class I epitopes (data not shown). These points show the high specificity of the ELISPOT assay.
Intracellular staining of IFN-γ.
Cells (1.8 × 106) were cultured for 6 h in 300 μl of complete medium with 10 μg of brefeldin A per ml in 12- by 75-mm test tubes, in the presence of various stimuli. The positive control consisted of mitogenic activation with phorbol myristate acetate (25 ng/ml) and ionomycin (1 μg/ml). Stimulator cells were autologous lymphoblastoid cell lines obtained by transforming PBMC with EBV (EBV-LCL). They were tested either untreated or after infection with vaccinia virus recombinants for various HIV-1LAI genes as described elsewhere (12). Reactivity against autologous EBV-LCL alone and against the same cell line infected with vaccinia virus recombinants expressing the env, gag, pol, and nef genes of HIV-1LAI were tested for each patient. Negative controls were medium alone and EBV-LCL infected with wild-type vaccinia viruses. The intracellular staining procedure of IFN-γ was adapted from the Becton Dickinson protocol. Cells were stained directly in the culture tubes with 15 μl of PerCP (peridinin chlorophyll a protein)-conjugated anti-CD8 and 15 μl of phycoerythrin CPE)-conjugated anti-CD28 or with isotype-matched negative control reagents. Cells were then permeabilized and incubated with 30 μl of anti-human IFN-γ-fluorescein isothiocyanate (FITC) or with isotype-matched negative control reagent. Finally, cells were washed, resuspended in 100 μl of cell wash containing 1% paraformaldehyde, and stored at 4°C in the dark for flow cytometry analysis.
Five-parameter analysis (forward and side scatter, FITC, PE, and PerCP) was performed on a FACScan flow cytometer using the Cellquest software (Becton Dickinson). For each sample, 125,000 to 800,000 events were acquired and gated on CD8 expression; a scatter gate was designed to include only viable lymphocytes.
Statistical analyses.
Data analyses were performed with the StatView 4.5 software (Abacus Concepts, Berkeley, Calif.). Comparisons between the anti-HIV and the anti-EBV responses were performed by using the Mann-Whitney test. Correlations between variables were identified by using simple linear regression analysis. Changes over time of CD4+ T-cell counts and intensity of antiviral CD8+ responses were summarized by the least-squares estimate of the slope of the linear regression plots of each measurement against time. The Wilcoxon rank test was used to compare the antiviral responses before and after antiretroviral therapy. P values of 0.05 or less were considered significant.
RESULTS
Comparison between the anti-HIV and the anti-EBV ex vivo CD8+ responses in unstimulated PBMC of HIV-seropositive individuals.
We compared the breadth and intensity of anti-HIV and anti-EBV ex vivo CD8+ T-cell responses in the natural history of HIV infection in a large population, 34 HIV-infected patients harboring at least 1 of 17 HLA alleles for which several HIV or EBV epitope peptides have been described (Table 1). At the time of the test, these patients had been diagnosed as HIV positive for an average of 4.8 years (range, 6 months to 12 years; median, 5 years), none had received HAART, and they harbored a wide range of CD4+ T-cell counts (27 to 952; mean, 395; median, 325) and viral loads (undetectable to 2,503,000; mean, 215,054; median, 36,000) (Table 2).
TABLE 2.
Characteristics of ex vivo functional CD8+ repertoire against HIV and EBV in 34 HIV-1-seropositive patients
| Patient | HLA | T-cell count (CD4/CD8)/ μl of blood | Plasma HIV RNA (copies/ml) | Positive HIV peptides
|
Positive EBV peptides
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency (no. of peptides recognized by patient/total no. tested) | No. of IFN-γ SFC/ 106 PBMC
|
Frequency (no. of peptides recognized by patient/total no. tested) | No. of IFN-γ SFC/ 106 PBMC
|
||||||||
| Totala | Meanb | Range | Total | Mean | Range | ||||||
| Z134 | A24/31, B35/40 | 27/825 | 316,000 | 5/11 | 2,020 | 404 | 100–905 | 1/2 | 80 | ||
| Z136 | A1/30, B8/13 | 30/355 | 2,503,000 | 4/12 | 3,015 | 753 | 60–2,370 | 0/2 | 0 | ||
| Z130 | A1/32, B8/27 | 67/1085 | 575,000 | 7/17 | 2,736 | 390 | 90–1,172 | 0/1 | 0 | ||
| Z001 | A2/24, B35/44 | 117/911 | NDc | 6/19 | 5,019 | 836 | 100–1,610 | 2/4 | 590 | 295 | 140–450 |
| C03M | A2/68, B15/35 | 157/1837 | 72,000 | 1/8 | 50 | 0/1 | 0 | ||||
| Z132 | A3/25, B18/61 | 163/1092 | 316,000 | 3/7 | 478 | 159 | 81–263 | ND | ND | ||
| C02M | A2/−, B44/51 | 190/857 | 36,000 | 1/10 | 230 | 1/3 | 80 | ||||
| P08 | A2/28, B35/53 | 201/821 | 15,800 | 2/11 | 3,416 | 1,708 | 416–3,000 | 2/2 | 196 | 98 | 53–143 |
| Z006 | A2/24, B7/37 | 220/1180 | ND | 3/11 | 4,551 | 1,517 | 186–3,163 | 1/2 | 132 | ||
| C017M | A3/30, B44/65 | 227/653 | 1,100 | 3/12 | 3,735 | 1,245 | 214–3,000 | 0/3 | 0 | ||
| P38 | A2/3, B7/55 | 249/1247 | ND | 8/20 | 6,407 | 800 | 25–2,610 | 2/2 | 242 | 121 | 38–204 |
| Z044 | A2/25, B18/44 | 291/834 | 0 | 5/16 | 9,071 | 1,814 | 368–6,700 | 1/3 | 58 | ||
| Z020 | A2/24, B18/44 | 293/1174 | ND | 5/15 | 2,793 | 558 | 28–1,716 | 1/3 | 64 | ||
| P04 | A1/2, B8/44 | 303/1272 | 440,000 | 3/15 | 726 | 242 | 80–410 | 1/1 | 27 | ||
| C014M | A3/31, B38/51 | 308/831 | 190 | 4/9 | 695 | 173 | 59–467 | ND | ND | ||
| Z129 | A2/11, B51/62 | 323/985 | 19,500 | 7/16 | 7,520 | 1,074 | 70–3,000 | 2/2 | 1,055 | 527 | 88–967 |
| Z131 | A3/31, B14/60 | 328/567 | 329,000 | 2/9 | 536 | 268 | 115–421 | ND | ND | ||
| P36 | A2/9, B12/12 | 329/988 | 76,000 | 4/13 | 4,401 | 1,100 | 441–1,516 | 0/3 | 0 | ||
| Z077 | A2/2, B7/50 | 334/1161 | 250,000 | 5/10 | 6,663 | 1,332 | 168–3,542 | 2/3 | 311 | 155 | 148–163 |
| Z118 | A24/−, B7/42 | 346/612 | 11,000 | 6/8 | 4,803 | 800 | 17–1,744 | 2/3 | 155 | 77 | 26–129 |
| C022M | A3/32, B7/62 | 476/537 | 27,000 | 6/14 | 841 | 140 | 6–449 | 2/2 | 180 | 90 | 18–162 |
| Z067 | A1/2, B7/8 | 490/5000 | ND | 8/18 | 16,413 | 2,051 | 120–7,260 | 0/3 | 0 | ||
| Z112 | A2/−, B7/61 | 513/ND | ND | 6/12 | 1,825 | 304 | 25–870 | 2/3 | 130 | 65 | 50–80 |
| Z050 | A1/3, B8/35 | 545/545 | 29,763 | 8/19 | 5,495 | 686 | 10–3,090 | 1/2 | 555 | ||
| Z028 | A11/23, B7/49 | 598/988 | 25,000 | 7/9 | 2,286 | 326 | 19–863 | 3/3 | 862 | 287 | 137–432 |
| P09 | A2/24, B12/17 | 600/844 | 112,000 | 4/14 | 1,542 | 385 | 25–1,100 | 1/3 | 331 | ||
| Z108 | A2/31, B44/44 | 666/1214 | 69,000 | 4/17 | 8,047 | 2,011 | 242–6,455 | 2/3 | 1,856 | 928 | 195–1661 |
| Z042 | A1/32, B7/8 | 709/2747 | 31,000 | 6/17 | 4,602 | 767 | 32–1,448 | 3/3 | 560 | 186 | 27–460 |
| P51 | A31/32, B8/27 | 787/1952 | 122,000 | 9/17 | 4,581 | 509 | 60–1,177 | 1/1 | 651 | ||
| Z100 | A2/32, B40/44 | 858/1201 | ND | 4/13 | 605 | 151 | 55–280 | 1/3 | 140 | ||
| Z038 | A3/11, B35/44 | 945/936 | 0 | 13/16 | 12,933 | 994 | 51–4,879 | 1/3 | 1,344 | ||
| Z059 | A11/30, B44/55 | 952/1224 | 0 | 6/13 | 1,391 | 231 | 32–755 | 2/4 | 165 | 82 | 15–150 |
| P01 | A1/9, B8/15 | ND/ND | ND | 5/11 | 4,796 | 1,128 | 149–2,223 | 1/1 | 898 | ||
| Z039 | A2/3, B27/38 | ND/ND | ND | 5/13 | 4,634 | 926 | 300–1,824 | ND | ND | ||
Sum of the numbers of SFC per 106 PBMC for all viral peptides recognized.
Total intensity divided by number of peptides recognized.
ND, not done.
The number of peptides tested for each patient (range, 7 to 20; median, 13) depended on each combination of HLA-A and HLA-B alleles. The positive responses of each individual are shown in Fig. 1. Three parameters were defined to describe the anti-HIV and anti-EBV CD8+ T-cell repertoires of each patient: (i) polyclonality (number of viral epitope peptides recognized), (ii) total intensity (sum of the numbers of antiviral CD8+ T cells per 106 PBMC for all viral peptides recognized), and (iii) mean intensity (total intensity divided by polyclonality) (Table 2).
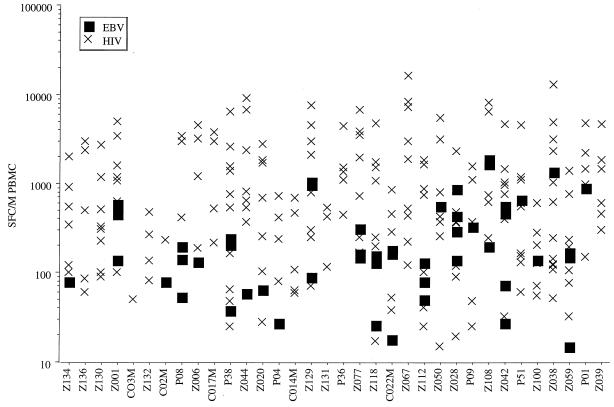
FIG. 1.
Intensity and polyclonality of anti-HIV and anti-EBV CD8+ responses of HIV-seropositive patients. The numbers of SFC per million (M) PBMC are shown for each patient and for each HIV and EBV peptide recognized.
All patients responded to HIV. Most recognized at least one HIV epitope for each HLA molecule tested. Anti-HIV responses showed great polyclonality (mean, 5; median, 5; range, 1 to 13), high total intensity (mean, 4,084; median, 3,576; range, 50 to 16,413), and high mean intensity (mean, 762; median, 720; range, 50 to 2,052).
Most (24 of 30) patients also responded to EBV with moderate total intensity (mean, 355; median, 160; range, 0 to 1,856) and moderate mean intensity (mean, 303; median, 136; range, 27 to 1,344). As fewer CD8+ epitopes have been described for EBV, the polyclonality (each patient recognized one EBV peptide on average [mean, 1; range, 0 to 3]), and total intensity of anti-EBV responses may be greatly underestimated. Therefore, with this experimental approach, it is not possible to compare the total intensity and polyclonality of the anti-EBV and anti-HIV repertoires. However, the mean intensity of anti-EBV responses was significantly lower than that of anti-HIV responses (Fig. 1; a mean of 303 SFC per 106 PBMC was obtained for each EBV peptide recognized, versus 762 for HIV [P < 0.0001]).
To compare the total intensities of anti-EBV and anti-HIV responses, we quantitated in six patients the CD8+ T lymphocytes capable to produce IFN-γ ex vivo in response to autologous EBV-LCL eventually infected with vaccinia virus recombinants carrying various HIV-1LAI genes, using intracellular staining and cytofluorometry. A much higher number of CD8+ T lymphocytes responded to stimulation with EBV-infected cells expressing also HIV-1 proteins than with cells expressing only EBV antigens (Table 3). We further verified the specificity of the responses observed by verifying that CD8+ T cells directed against HIV were mostly CD28− whereas those responding to EBV-LCL alone or infected with wild-type vaccinia virus were mostly CD28+, as previously reported (13). Therefore, the total frequency of anti-HIV CD8+ T-cell responses appears much more important than that of anti-EBV responses, at least in the six subjects tested.
TABLE 3.
Analysis of ex vivo CD8+ T-cell responses specific for autologous EBV-LCL eventually infected with vaccinia virus recombinants for various HIV-1LAI genes
| Patient | Medium
|
EBV
|
Vaccinia virus
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequencya | CD28b | Frequency | CD28 | Wild type
|
Env
|
Gag
|
Pol
|
Nef
|
||||||
| Frequency | CD28 | Frequency | CD28 | Frequency | CD28 | Frequency | CD28 | Frequency | CD28 | |||||
| C03M | 202 | 65 | 1,161 | 73 | 883 | 71 | 6,244 | 24 | 4,728 | 31 | 4,450 | 23 | 3,914 | 28 |
| P04 | 258 | 22 | 2,142 | 20 | 1,663 | 24 | 7,674 | 12 | 3,296 | 16 | 2,555 | 18 | 1,282 | 21 |
| Z050 | 87 | 69 | 1,572 | 71 | 868 | 59 | 1,141 | 41 | 1,970 | 29 | 2,270 | 26 | 2,866 | 37 |
| Z077 | 224 | 15 | 3,494 | 60 | 1,749 | 52 | 7,726 | 15 | 7,492 | 21 | 18,000 | 10 | 5,060 | 27 |
| Z100 | 188 | 26 | 5,976 | 50 | 2,810 | 42 | 6,018 | 18 | 20,225 | 16 | 9,825 | 22 | 2,551 | 38 |
| Z108 | 333 | 62 | 1,392 | 74 | 760 | 62 | 1,147 | 39 | 7,867 | 17 | 2,960 | 27 | 923 | 48 |
Number of CD8+ T cells secreting IFN-γ in response to antigen per 106 total CD8+ lymphocytes.
Percent CD28 expression on IFN-γ+ CD8+ T lymphocytes. Generally both a higher frequency of IFN-γ-secreting cells and a lower expression of CD28 attest to the specificity of the responses directed against EBV-LCL infected with vaccinia virus recombinants for HIV genes compared with those against EBV-LCL infected with wild-type vaccinia virus (values in boldface).
The data suggested massive ex vivo HIV-specific CD8+ T-cell responses, as well as the persistence of weaker anti-EBV responses, at all stages of HIV infection. We therefore examined the influence of HIV-induced immunodepression on anti-HIV and anti-EBV responses and the relationship between anti-HIV responses and virus load.
Influence of HIV-induced immunodepression on antiviral CD8+ ex vivo responses.
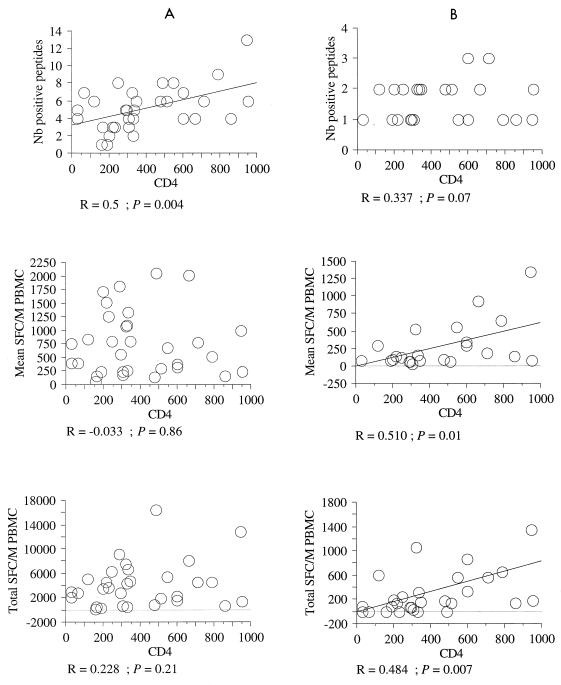
The CD4+ T-cell counts were used as a marker of HIV-induced immunodepression, and their relationship with antiviral responses was examined (Fig. 2). The polyclonality of anti-HIV responses was positively correlated with the CD4+ T-cell counts. Neither the mean intensity nor the total intensity of anti-HIV responses was correlated with the CD4+ T-cell counts, even in comparisons of patients for the reactivity with a given HLA molecule (not shown). In contrast, the total intensity and mean intensity of anti-EBV CD8+ responses were positively correlated with CD4+ T-cell counts. Therefore, unlike the anti-HIV responses, the anti-EBV responses tended to decrease with increasing HIV-1-induced immunodepression.
FIG. 2.
Relationship between CD4+ T-cell counts and anti-HIV or anti-EBV CD8+ responses of HIV-seropositive patients. The antiviral repertoire of each patient was analyzed on the basis of three parameters, polyclonality, total intensity, and mean intensity, as described in the text. Each of these parameters of anti-HIV (A) or anti-EBV (B) CD8+ responses was expressed as a function of CD4+ T-cell counts. A linear regression was established by using StatView 4.5 software (Abacus Concepts). Only the statistically significant linear regressions are drawn. M, million.
Relationship between virus load and anti-HIV ex vivo CD8+ responses.
The polyclonality and mean intensity of anti-HIV CD8+ T-cell responses were apparently not correlated with virus load (r = −0.312, P = 0.13; and r = −0.223, P = 0.28, respectively). However, there was a trend toward an inverse relationship between the total intensity of anti-HIV CD8+ T-cell responses and virus load, although the correlation was not significant (r = −0.381, P = 0.06). There was also no clear correlation between virus load and anti-HIV ex vivo CD8+ T-cell responses in comparisons of patients for reactivity with a given HLA molecule (not shown).
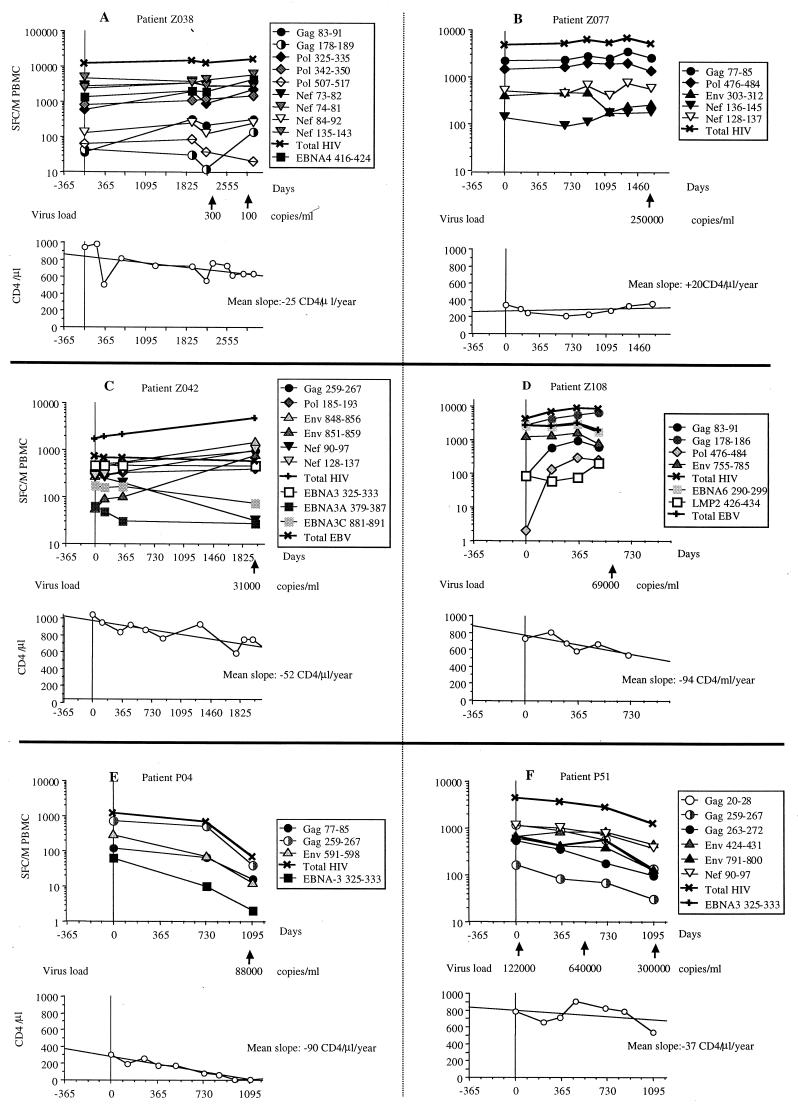
Evolution of IFN-γ secretion in untreated seropositive individuals.
Six of the 34 HIV-seropositive patients studied were followed longitudinally for several years while they received no efficient antiretroviral therapy (Fig. 3). Patients Z038 and Z077 had very stable anti-HIV and anti-EBV responses against all tested epitopes over 8 and 5 years, respectively (Fig. 3A and B). These patients also had remarkably stable CD4+ T-cell counts during that period. Patients Z042 and Z108 showed increases in anti-HIV CD8+ T-cell responses, while their anti-EBV responses were consistently stable or decreased moderately (Fig. 3C and D). The CD4+ T-cell counts of these patients clearly decreased during that period. Finally, patients P04 and P51 showed significant decreases in anti-HIV and anti-EBV CD8+ T-cell responses (Fig. 3E to F). Patient P04 had a dramatic decrease in CD4+ T-cell counts during follow-up (Fig. 3E). This subject was symptomatic at enrollment and died of AIDS 3 years later. By contrast, patient P51 showed only a moderate decrease of CD4+ T-cell counts, even smaller than that of patients Z042 and Z108. However, this subject had a very high virus load, which increased sharply from 120,000 to 600,000 copies of HIV RNA per ml of plasma in the first 1.5 years of study (Fig. 3F).
FIG. 3.
Longitudinal analysis of ex vivo antiviral CD8+ responses of six untreated HIV-seropositive individuals. CD8+ T cells specific for individual HIV-1 (circles, triangles, and diamonds) and EBV (squares) epitope peptides were quantified longitudinally in six individuals who were not receiving HAART during the period of observation. Total anti-HIV (X) and anti-EBV (+) responses calculated as the sum of the SFC numbers for all peptides recognized are also indicated. Values of virus load when available (arrows), evolution of CD4+ cell counts, and mean slope of CD4+ decline for the entire period of observation are also shown for each patient.
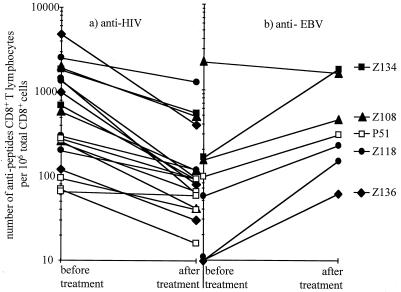
Impact of HAART on antiviral CD8+ T-cell ex vivo responses.
We also studied the impact of HAART on the numbers of anti-HIV and anti-EBV CD8+ T cells in five patients treated during the asymptomatic phase of HIV-1 infection. Patients Z108, Z118, Z134, and Z136 were given triple combination therapy, with a protease inhibitor and two nucleoside reverse transcriptase inhibitors, while patient P51 was treated with two nucleoside reverse transcriptase inhibitors. Both treatment regimens rapidly reduced plasma HIV RNA to undetectable levels. There were significant decreases in anti-HIV CD8+ T-cell responses in all patients against all HIV epitopes initially recognized (Fig. 4A; P = 0.0003). The anti-EBV CD8+ T-cell responses increased in all patients (Fig. 4B).
FIG. 4.
Impact of HAART on antiviral CD8+ T-cell responses. The frequency of CD8+ T cells specific for HIV (a) and EBV (b) epitope peptides is shown just before initiation of HAART and after reduction of viral load to undetectable levels in the five patients noted at the right.
DISCUSSION
Several authors have proposed that the intense recognition of many HIV epitope peptides is specific to slow progressors or nonprogressors (4, 22, 30, 31, 36) and is important in controlling virus replication efficiently. In contrast, the present analysis of the ex vivo antiviral CD8+ T-cell repertoires of 34 HIV-seropositive patients revealed that intense recognition of many HIV-1 peptides is a common feature of anti-HIV responses, regardless of the CD4+ T-cell counts or virus load. Indeed, most individuals had a broad repertoire of anti-HIV CD8+ T cells, since each patient responded to many HIV epitopes, with high frequencies of CD8+ T cells against many of them, often in association with all HLA class I molecules tested. Many of these peptides still yielded significant numbers of SFC at doses as low as 10−9 M (data not shown). The breadth of the anti-HIV CD8+ repertoire revealed by the ELISPOT assay in these 34 seropositive individuals is all the more impressive in that it must be greatly underestimated, for three reasons. First, the HIV CD8+ epitopes have been extensively defined for only a few HLA alleles, so that the number of potential epitopes that we tested was restricted in some patients with nonclassical HLA alleles. Second, since the peptides synthesized were based on the HIV-1LAI sequence, all epitopes found are probably highly conserved among most HIV-1 isolates (this is particularly important since there may be far more CD8+ T cells directed against epitopes specific to autologous virus than CD8+ T cells directed against conserved epitopes [9, 16, 19]). Finally, more than one CD8+ T-cell clone may be used for each peptide recognized by a given patient (31, 39).
As it is clearly established that anti-HIV CD8+ responses are a major factor in the control of viral replication, it may seem surprising that we did not observe a clear negative correlation between viral load and the frequency of anti-HIV CD8+ T-cell responses (r = −0.381; P = 0.06; not significant), in contrast with the recent observations by Ogg et al. (35). The fact that this correlation did not reach significance may be related to the relatively low number of patients with known levels of plasma HIV RNA (n = 25) or to a shift in the distribution of viral loads toward high values (only five patients harbored plasma HIV RNA levels below 10,000 copies/ml), both of which can reduce the power of statistical analysis.
However, the relationship between viral replication and anti-HIV CD8+ T-cell responses is very complex. Despite harboring intense anti-HIV CD8+ T-cell responses, some patients harbor relatively high levels of viral replication (patients Z050, Z077, Z108, and Z129 of our study, for example; see also reference 6). Whereas subject Z067 harbored one of the most intense anti-HIV responses, he evolved toward disease very rapidly and died of AIDS within 2 years of infection. Moreover, we and others have clearly shown that patients with very low viral load may have also very low frequencies of anti-HIV CD8+ T cells, likely because of low levels of in vivo antigen-specific stimulation (patients C014M and Z059 of our study; references 3, 11, 14, and 17). Therefore, in most patients a minimal level of viral replication seems to be needed for maintenance of a high frequency of anti-HIV CD8+ T cells, as suggested by our analysis of the impact of HAART on antiviral responses. Finally, the fact that the total frequency of anti-HIV CD8+ T cells did not correlate significantly with viral load is consistent with the observation that it did not correlate either with CD4+ counts.
We also tested the seropositive patients for reactivity against several EBV CD8+ epitopes described as immunodominant. EBV antigens were widely recognized, as 24 of 30 patients responded against at least one EBV peptide. These results agree with data from other studies using LDA that reported no significant change in anti-EBV responses over the course of HIV infection but a decrease in anti-HIV CTL responses late in the development of AIDS (10, 23). We found a significantly lower mean intensity against individual EBV peptides than against HIV peptides. Moreover, in six patients we observed a much higher number of CD8+ T lymphocytes responding to stimulation with EBV-infected cells expressing also HIV-1 proteins than with cells expressing only EBV antigens. We believe that these experiments give a good picture of the total intensity of anti-EBV responses. In contrast, the total frequency of anti-HIV CD8+ T cells must still be underestimated, because all HIV proteins were not tested as target antigens and because all vaccinia virus-expressed proteins derived from HIV-1LAI and not from autologous virus. Thus, these results strongly argue for a much lower intensity of anti-EBV than of anti-HIV CD8+ T-cell responses, which may also suggest a lower polyclonality.
The difference in intensity between the anti-HIV and the anti-EBV responses in the HIV-seropositive patients may be due to a difference in balance between the immunosuppressive effect of deficient CD4+ T-cell help and the immunoactivatory effect of antigenic stimulation, linked to the different levels of replication of these viruses in vivo. The high replication of HIV in vivo should generate intense specific activation of CD8+ T cells which may partially offset the immunosuppressive effect of CD4+ T-cell depletion. Indeed, the intensity of anti-EBV CD8+ responses was positively correlated with the CD4+ T-cell counts, while the intensity of anti-HIV responses was not. However, the polyclonality of anti-HIV CD8+ T-cell responses was positively correlated with the CD4+ T-cell counts. These observations are in agreement with recent longitudinal studies by LDA showing that total numbers of anti-HIV CD8+ T cells are not correlated with clinical evolution whereas anti-Gag responses are (37). This finding suggests that the anti-EBV CD8+ T-cell responses of HIV-seropositive patients depend more on CD4+ T-cell help than do anti-HIV responses and that highly polyclonal anti-HIV CD8+ T-cell responses are associated with better clinical status, probably by avoiding selection of virus CTL-escape mutants.
Since we observed a positive correlation between CD4+ T-cell counts and the polyclonality of anti-HIV CD8+ T-cell frequencies, one might have expected a negative correlation between the latter parameter and viral load, as CD4+ T-cell counts correlate negatively with plasma HIV RNA, though weakly, in our study (log viral load versus CD4+ counts, r = −0.002, P = 0.05). However, this was not the case (polyclonality of anti-HIV CD8+ T-cell frequencies versus log viral load, r = −0.312, P = 0.13). Perhaps the size of our cohort is too low to address the relationship between viral load and other parameters, especially considering the shift of the distribution of viral loads toward high values, as mentioned above. However, it seems that CD4+ T-cell counts may be a better marker of clinical status than viral load in our patients, as some of them with low CD4+ T-cell counts have relatively moderate levels of plasma HIV RNA despite receiving no HAART (patients C03M, C02M, P08, and C017M, for example) but eventually harbor clinical symptoms of disease (patients C03M and P08). They all show low polyclonality of CD8+ T-cell responses. Other patients harboring similar or higher levels of plasma HIV RNA have higher CD4+ T-cell counts that seem to be associated with a broader repertoire of anti-HIV CD8+ T cells and higher levels of anti-EBV CD8+ T-cell responses (patients Z050, Z028, P09, Z108, Z042, and P51).
We further investigated the relationship between antiviral CD8+ responses and CD4+ T-cell counts by longitudinal follow-up of several patients. There were three patterns of anti-HIV CD8+ responses, which seemed to be associated with different patterns of evolution of anti-EBV CD8+ responses and CD4+ T-cell counts. Two patients had stable antiviral CD8+ responses and stable T-cell counts throughout the period of observation. One of these patients had a persistently very low to undetectable virus load, while the other had around 250,000 copies HIV RNA per ml of plasma. This finding suggests that they had reached a specific equilibrium between virus replication and the host immune response, with sufficient control of HIV replication to avoid major immunodepression. In contrast, two other patients showed increases in anti-HIV CD8+ responses, while their anti-EBV CD8+ responses remained stable or decreased moderately and their CD4+ T-cell counts decreased significantly, which led to prescription of HAART. The specific increases in anti-HIV CD8+ responses in these patients probably reflect increased HIV replication in vivo which had not yet reached the threshold for the strong immunodepression that significantly reduces anti-EBV CD8+ responses. Finally, two patients had a clear reduction in the numbers of anti-HIV and anti-EBV CD8+ T cells over time. This was associated with a drastic reduction in CD4+ T-cell counts and ultimately in one patient with death from AIDS and in the other with a sharp increase in virus load that led to the prescription of HAART, although the CD4+ T-cell counts had decreased only moderately. In these patients, it is probable that very high HIV-1 replication over a long period caused massive immunodepression that could no longer be counterbalanced by antigen-specific activation, and so there was a significant decrease in anti-HIV CD8+ responses in parallel with the anti-EBV responses.
The relationship between virus load and maintenance of a strong immune response was studied in five patients receiving HAART. Treatment resulted in a significant decrease in the number of anti-HIV CD8+ T cells in all individuals, with a parallel reduction in plasma HIV RNA to undetectable levels. These results obtained during the chronic phase of HIV infection confirm similar observations made in studies using a classical chromium release test after in vitro stimulation among a large cohort of seroconverters (12). Ogg et al. found a similar decrease in CTLe frequency in treated asymptomatic patients, using HLA peptide tetramers (35). The decrease in CTLe frequency could be due to a decrease in virus replication or to a direct immunosuppressive effect of the antiretroviral therapy. The immunosuppressive effect is unlikely, since the patients that we studied increased their CD8+ T-cell responses against EBV during HAART. The fact that HAART improved the anti-EBV response confirms that some immunosuppression of anti-EBV CD8+ responses may occur early in asymptomatic patients and increases with CD4+ T-cell depletion as discussed above but can be restored, at least partially, under triple combination therapy.
Our findings emphasize the magnitude of HIV-specific CD8+ T-cell responses in seropositive individuals at all stages of HIV infection. The ELISPOT assay gave frequencies of anti-HIV and anti-EBV epitope-specific CD8+ T cells that were significantly higher than those reported for classical LDA experiments (5, 18, 21, 24, 28), agreeing with recent reports in which HLA peptide tetramers were used to study CD8+ T-cell responses in human, simian, and murine infections (1, 8, 25, 34). However, although significantly higher than anti-EBV values, the percentages of anti-HIV CD8+ T cells reported in our study, as in that of Ogg et al. (35), are still far below the unusually high percentages of activated CD8+ T cells expressing CD38 commonly observed in HIV infection. We believe that the total number of anti-HIV CD8+ T cells that we obtained was greatly underestimated, due to various sources of experimental bias. Thus, it is highly probable that the CD8+ T-cell hyperlymphocytosis commonly observed in HIV infection is mainly driven by virus replication, through intense activation of HIV-specific CD8+ T cells. The specific decrease in the number of anti-HIV CD8+ T cells in patients on HAART strongly supports this hypothesis. If nonspecific bystander activation due to general inflammation plays an important role in the activation of CD8+ T lymphocytes observed in HIV-1-infected patients, there should be an increase in both anti-EBV and anti-HIV responses during HIV infection and hence a decrease in both responses under HAART as the inflammation is reduced. But we find an increase in anti-EBV CD8+ responses in several patients on HAART. Furthermore, we have recently shown that anti-EBV CD8+ T cells have a clearly less activated phenotype ex vivo than do anti-HIV CD8+ T cells in HIV-seropositive individuals (13). These observations confirm a recent analyses of the kinetic of antiviral CD8+ T-cell responses in natural acute EBV infection in humans (8) and during experimental lymphocytic choriomeningitis virus infection in mice (34), which showed that the antigen-specific activation of CD8+ effector cells is much greater than nonspecific bystander activation.
ACKNOWLEDGMENTS
We thank all subjects who donated blood for our study. We also are grateful to Jeannine Choppin, Béatrice Culmann-Penciolelli, and Elisabeth Gomard for providing records of optimal epitopes described for HIV and EBV, as well as to François Dreyfus and Jean-Marc Bouley for centralizing the clinical data for some patients. The English text was edited by Owen Parkes.
This work was supported in part by grants from the Agence Nationale de la Recherche sur le SIDA and Ensemble contre le Sida, Sidaction, France. Marc Dalod is supported by a fellowship from the Ministère de l’Enseignement Supérieur, de la Recherche, et des Techniques, Government of France.
REFERENCES
- 1.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Bariou C, Genetet N, Ruffault A, Michelet C, Cartier F, Genetet B. Longitudinal study of HIV-specific cytotoxic T lymphocyte in HIV type 1 infected patients: relative balance between host immune response and the spread of HIV type 1 infection. AIDS Res Hum Retroviruses. 1997;13:1301–1312. doi: 10.1089/aid.1997.13.1301. [DOI] [PubMed] [Google Scholar]
- 3.Binley J M, Jin X, Huang Y, Zhang L, Cao Y, Ho D D, Moore J P. Persistent antibody response but declining cytotoxic T-lymphocyte responses to multiple human immunodeficiency virus type 1 antigens in a long-term nonprogressing individual with a defective p17 proviral sequence and no detectable viral RNA expression. J Virol. 1998;72:3472–3474. doi: 10.1128/jvi.72.4.3472-3474.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B A, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 5.Bourgault I, Gomez A, Gomard E, Levy J P. Limiting-dilution analysis of the HLA restriction of anti-Epstein-Barr virus-specific cytolytic T lymphocytes. Clin Exp Immunol. 1991;84:501–507. [PMC free article] [PubMed] [Google Scholar]
- 6.Brander C, Goulder P J R, Luzuriaga K, Yang O O, Hartman K E, Jones N G, Walker B D, Kalams S A. Persistent HIV-1-specific CTL clonal expansion despite high viral burden post in utero HIV-1 infection. J Immunol. 1999;162:4796–4800. [PubMed] [Google Scholar]
- 7.Brinchman J E, Dobloug J H, Heger B H, Haaheim L L, Sannes M, Egeland T. Expression of costimulatory molecule CD28 on T cells in human immunodeficiency virus type 1 infection: functional and clinical correlations. J Infect Dis. 1994;169:730–738. doi: 10.1093/infdis/169.4.730. [DOI] [PubMed] [Google Scholar]
- 8.Callan M F C, Tan L, Annels N, Ogg G S, Wilson J D K, O’Callaghan C A, Steven N, McMichael A J, Rickinson A B. Direct visualisation of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmichael A, Jin X, Sissons P. Analysis of the human env-specific cytotoxic T-lymphocyte (CTL) response in natural human immunodeficiency virus type 1 infection: low prevalence of broadly cross-reactive env-specific CTL. J Virol. 1996;70:8468–8476. doi: 10.1128/jvi.70.12.8468-8476.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalod M, Fiorentino S, Delamare C, Rouzioux C, Sicard D, Guillet J G, Gomard E. Delayed virus-specific CD8+ cytotoxic T lymphocyte activity in an HIV-infected individual with high CD4+ cell counts: correlations with various parameters of disease progression. AIDS Res Hum Retroviruses. 1996;12:497–506. doi: 10.1089/aid.1996.12.497. [DOI] [PubMed] [Google Scholar]
- 12.Dalod M, Harzic M, Pellegrin I, Dumon B, Hoen B, Sereni D, Deschemin J-C, Levy J-P, Venet A, Gomard E. Evolution of cytotoxic T lymphocyte responses to HIV-1 in patients with symptomatic primary infection on antiretroviral triple therapy. J Infect Dis. 1998;178:61–69. doi: 10.1086/515587. [DOI] [PubMed] [Google Scholar]
- 13.Dalod M, Sinet M, Deschemin J, Fiorentino S, Venet A, Guillet J. Altered ex vivo balance between CD28+ and CD28− cells within HIV-specific CD8+ T cells of HIV-seropositive patients. Eur J Immunol. 1999;29:38–44. doi: 10.1002/(SICI)1521-4141(199901)29:01<38::AID-IMMU38>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Ferbas J, Kaplan A H, Hausner M A, Hultin L E, Matud J L, Liu Z, Panicali D L, Nerng-Ho H, Detels R, Giorgi J V. Virus burden in long-term survivors of human immunodeficiency virus infection is a determinant of anti-HIV CD8+ lymphocyte activity. J Infect Dis. 1995;172:329–339. doi: 10.1093/infdis/172.2.329. [DOI] [PubMed] [Google Scholar]
- 15.Giorgi J V, Liu Z, Hultin L E, Cumberland W G, Hennessey K, Detels R. Elevated levels of CD38+CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. J Acquired Immune Defic Syndr. 1993;6:904–912. [PubMed] [Google Scholar]
- 16.Goulder P, Price D, Nowak M, Rowland-Jones S, Phillips R, McMichael A. Co-evolution of human immunodeficiency virus and cytotoxic T-lymphocyte responses. Immunol Rev. 1997;159:17–29. doi: 10.1111/j.1600-065x.1997.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 17.Greenough C, Somasundaran M, Brettler D B, Hesselton R M, Alimenti A, Kirchhoff F, Panicali D, Sullivan J L. Normal immune function and inability to isolate virus in culture in an individual with long-term human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 1994;4:395–403. doi: 10.1089/aid.1994.10.395. [DOI] [PubMed] [Google Scholar]
- 18.Greenough T C, Brettler D B, Somasundaran M, Panicali D L, Sullivan J L. Human immunodeficiency virus type-1-specific cytotoxic T lymphocytes, virus load, and CD4 T cell loss: evidence supporting a protective role for CTL in vivo. J Infect Dis. 1997;176:118–125. doi: 10.1086/514013. [DOI] [PubMed] [Google Scholar]
- 19.Haas G, Plikat U, Debré P, Lucchiari M, Katlama C, Dudoit Y, Bonduelle O, Bauer M, Ihlenfeldt H-G, Jung G, Maier B, Meyerhans A, Autran B. Dynamics of viral variants in HIV-1 Nef and specific cytotoxic T lymphocytes in vivo. J Immunol. 1996;157:4212–4221. [PubMed] [Google Scholar]
- 19a.HIV Molecular Immunology Database.http://hiv-web.lanl.gov/immuno/index. [Online.]
- 20.Ho H N, Hultin L E, Mitsuyasu R T, Matud J L, Hausner M A, Bockstoce D, Chou C-C, O’Rourke S, Taylor J M G, Giorgi J V. Circulating HIV-specific CD8+ cytotoxic cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–3079. [PubMed] [Google Scholar]
- 21.Hoffenbach A, Langlade-Demoyen P, Dadaglio G, Vilmer E, Michel F, Mayaud C, Autran B, Plata F. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J Immunol. 1989;142:452–462. [PubMed] [Google Scholar]
- 22.Kalams S A, Johnson R P, Trocha A K, Dynan M J, Ngo H S, D’Aquila R T, Kurnick J T, Walker B D. Longitudinal analysis of T cell receptor (TCR) genes usage by human immunodeficiency virus 1 envelope-specific cytotoxic T lymphocyte clones reveals a limited TCR repertoire. J Exp Med. 1994;179:1261–1271. doi: 10.1084/jem.179.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kersten M J, Klein M R, Holwerda A M, Miedema F, van Oers M H J. Epstein-Barr virus-specific cytotoxic T cell responses in HIV-1 infection. Different kinetics in patients progressing to opportunistic infection or non-Hodgkin’s lymphoma. J Clin Investig. 1997;99:1525–1533. doi: 10.1172/JCI119315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koup R A, Pikora C A, Luzuriaga K, Brettler D B, Day E S, Mazzara G P, Sullivan J L. Limiting dilution analysis of cytotoxic T lymphocytes to human immunodeficiency gag antigens in infected persons: in vitro quantitation of effector cell populations with p17 and p24 specificities. J Exp Med. 1991;174:1593–1600. doi: 10.1084/jem.174.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuroda M J, Scmitz J E, Barouch D H, Craiu A, Allen T M, Sette A, Watkinc D I, Forman M A, Letvin N L. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class-I-peptide complex. J Exp Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V S, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lalvani A, Brookes R, Wilkinson R J, Malin A S, Pathan A A, Andersen P, Dockrell H, Pasvol G, Hill A V S. Human cytolytic and interferon-γ secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–275. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamhamedi-Cherradi S, Culmann-Penciolelli B, Guy B, Kieny M-P, Dreyfus F, Saimot A-G, Sereni D, Sicard D, Levy J-P, Gomard E. Qualitative and quantitative analysis of human cytotoxic T-lymphocyte responses to HIV-1 proteins. AIDS. 1992;6:1249–1258. doi: 10.1097/00002030-199211000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Levy J-P. Questions about CD8+ anti-HIV lymphocytes in the control of HIV infection. Antibiot Chemother. 1996;48:13–20. doi: 10.1159/000425153. [DOI] [PubMed] [Google Scholar]
- 30.Liebermann J, Fabry J A, Kuo M C, Earl P, Moss B, Skolnik P R. Cytotoxic T lymphocytes from HIV-1 seropositive individuals recognize immunodominant epitopes in Gp160 and reverse transcriptase. J Immunol. 1992;148:2738–2747. [PubMed] [Google Scholar]
- 31.Lubaki N M, Ray S C, Dhruva B, Quinn T C, Siliciano R F, Bollinger R C. Characterization of a polyclonal cytolytic T lymphocyte response to human immunodeficiency virus in persons without clinical progression. J Infect Dis. 1997;175:1360–1367. doi: 10.1086/516468. [DOI] [PubMed] [Google Scholar]
- 32.Matano T, Shibata R, Siemon C, Connors M, Lane M C, Martin M A. Administration of an anti-CD8 monoclonal antibody interferes with viral clearance of the chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.MHCPEP Database. gopher://wehll.wehi.edu.au:70/77/MHCPEP.DB. [Online.]
- 33.Moss P A, Rowland-Jones S, Frodsham P M, McAdam S, Giangrandes P, McMichael A, Bell J I. Persistent high frequency of human immunodeficiency virus-specific cytotoxic T cells in the peripheral blood of infected donors. Proc Natl Acad Sci USA. 1995;92:5773–5777. doi: 10.1073/pnas.92.13.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 35.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma viral load RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 36.Pantaleo G, Demarest J F, Schacker T, Vaccarezza M, Cohen O J, Daucher M, Graziosi C, Schnittman S S, Quinn T C, Shaw G M, Perrin L, Tambussi G, Lazzarin A, Sekaly R P, Soudeyns H, Corey L, Fauci A S. The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia. Proc Natl Acad Sci USA. 1997;94:254–258. doi: 10.1073/pnas.94.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pontesilli O, Klein M R, Kerkhof-Garde S R, Pakker N G, de Wolf F, Schuitemaker H, Miedema F. Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant gag-specific response is associated with nonprogressive infection. J Infect Dis. 1998;178:1008–1018. doi: 10.1086/515659. [DOI] [PubMed] [Google Scholar]
- 38.Rinaldo C R, Belz L A, Huang X-L, Gupta P, Fan Z, Torpey D J. Anti-HIV type 1 cytotoxic T lymphocyte effector activity and disease progression in the first 8 years of HIV type 1 infection of homosexual men. AIDS Res Hum Retroviruses. 1995;11:481–489. doi: 10.1089/aid.1995.11.481. [DOI] [PubMed] [Google Scholar]
- 39.Safrit J T, Andrews C A, Zhu T, Ho D D, Koup R A. Characterization of human immunodeficiency virus type-1 specific cytotoxic T lymphocyte clones isolated during acute seroconversion: recognition of autologous virus sequences within a conserved immunodominant epitope. J Exp Med. 1994;179:463–472. doi: 10.1084/jem.179.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheibenbogen C, Lee K-H, Stevanovic S, Witzens M, Willhauck M, Waldmann V, Naeher H, Rammensee H-G, Keilholz U. Analysis of the T cell response to tumor and viral peptide antigens by an IFN-γ ELISPOT assay. Int J Cancer. 1997;71:932–936. doi: 10.1002/(sici)1097-0215(19970611)71:6<932::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 42.Trimble L A, Lieberman J. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downmodulate CD3zeta, the signaling chain of the T-cell receptor complex. Blood. 1998;91:585–594. [PubMed] [Google Scholar]