Trazodone
 | |
 | |
| Names | |
|---|---|
| Trade names | Many brand names worldwide[1] |
| Other names | AF-1161 |
| |
| Clinical data | |
| Dependence risk | None[4] |
| Addiction risk | None[4] |
| Pregnancy category |
|
| Routes of use | By mouth (tablets) |
| Onset of action | By mouth: 1 hour (Tmax)[2] |
| Defined daily dose | 300 mg[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681038 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | By mouth: 65%[5] |
| Protein binding | 89–95%[6] |
| Metabolism | Liver (CYP3A4)[7] |
| Metabolites | mCPP[8] |
| Elimination half-life | Trazodone IR: 7 hours[5] Trazodone ER: 10 hours[5] mCPP: 4–8 hours[9] |
| Excretion | Urine: 70–75%[5] Feces: 21%[5] |
| Chemical and physical data | |
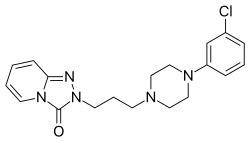
| Formula | C19H22ClN5O |
| Molar mass | 371.87 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 87 °C (189 °F) |
| |
| |
Trazodone, sold under many brand names,[1] is an antidepressant medication.[10] It is used to treat major depressive disorder, anxiety disorders, trouble sleeping and, with other medications, alcohol dependence.[10][11] It is taken by mouth.[10]
Common side-effects include dry mouth, feeling faint, vomiting, and headache.[10] More serious side effects may include suicide, mania, irregular heart rate, and pathologically prolonged erections.[10] It is unclear if use during pregnancy or breastfeeding is safe.[12] It is a phenylpiperazine compound of the serotonin antagonist and reuptake inhibitor (SARI) class.[13][14] Trazodone also has sedating effects.[15]
Trazodone was approved for medical use in the United States in 1981.[10] It is available as a generic medication.[10] The cost in the United Kingdom for the NHS is about £7.46 per month as of 2019.[16] In the United States, the wholesale cost is about US$4.53 per month as of 2018.[17] In 2017, it was the 30th most commonly prescribed medication in the United States, with more than 22 million prescriptions.[18][19]
Medical uses
Trazodone has the following medical uses:
- Unipolar depression, with or without anxiety[20]
- Anxiety disorder[21]
- Insomnia[22]
Depression
The primary use of trazodone is the treatment of major depression. Data from open and double-blind trials suggest the antidepressant efficacy of trazodone is comparable to that of amitriptyline, doxepin, and mianserin. Also, trazodone showed anxiolytic properties, low cardiotoxicity, and relatively mild side effects.[23]
Because trazodone has minimal anticholinergic activity, it was especially welcomed as a treatment for geriatric patients with depression when it first became available. Three double-blind studies reported trazodone has antidepressant efficacy similar to that of other antidepressants in geriatric patients. However, a side effect of trazodone, orthostatic hypotension, which may cause dizziness and increase the risk of falling, can have devastating consequences for elderly patients; thus, this side effect, along with sedation, often makes trazodone less acceptable for this population, compared with newer compounds that share its lack of anticholinergic activity but not the rest of its side-effect profile. Still, trazodone is often helpful for geriatric patients with depression who have severe agitation and insomnia.[23]
Insomnia
Low-dose trazodone is an alternative to benzodiazepines for the treatment of insomnia. Two reviews found that it is the second most prescribed agent for insomnia, but as most studies have been limited to people with depression, few studies actually support trazodone's use in primary insomnia.[23] It may decrease episodes of awaking by 0.3 per night.[11] General use; however, is not recommended for insomnia as of 2021.[11]
Non-approved
Off-label uses and investigational uses are listed below:
- Complex regional pain syndrome[24]
- Obsessive–compulsive disorder (OCD)[25]
- Alcohol withdrawal[26][27][28]
- Schizophrenia as an adjunct to improve negative symptoms.[29]
- Erectile dysfunction[30]
Dosage
The defined daily dose is 300 mg by mouth.[3]
Trazodone is available in the form of 25 mg, 50 mg, 100 mg, 150 mg, and 300 mg tablets for oral ingestion.[31][32]
An extended release formulation at 150 mg and 300 mg as tablets is also available.[33][34]
Side effects
Because of its lack of anticholinergic side effects, trazodone is especially useful in situations in which antimuscarinic effects are particularly problematic (e.g., in patients with benign prostatic hyperplasia, closed-angle glaucoma, or severe constipation). Trazodone's propensity to cause sedation is a dual-edged sword. For many patients, the relief from agitation, anxiety, and insomnia can be rapid; for other patients, including those individuals with considerable psychomotor retardation and feelings of low energy, therapeutic doses of trazodone may not be tolerable because of sedation. Trazodone elicits orthostatic hypotension in some people, probably as a consequence of α1-adrenergic receptor blockade. The unmasking of bipolar disorder may occur with trazodone[10] and other antidepressants.[35]
Precautions for trazodone include known hypersensitivity to trazodone and under 18 years and combined with other antidepressant medications, it may increase the possibility of suicidal thoughts or actions.[36]
Trazodone has been reported to cause seizures in a small number of patients who took it concurrently with medications to control seizures.
While trazodone is not a true member of the SSRI class of antidepressants, it does still share many properties of the SSRIs, especially the possibility of discontinuation syndrome if the medication is stopped too quickly.[37] Care must, therefore, be taken when coming off the medication, usually by a gradual process of tapering down the dose over a period of time.
Suicide
Antidepressants increase the risk of suicidal thoughts and behaviors in children and young adults. Close monitoring for emergence of suicidal thoughts and behaviors is thus recommended.[38]
Sedation
Since trazodone may impair the mental and/or physical abilities required for performance of potentially hazardous tasks, such as operating an automobile or machinery, the patient should be cautioned not to engage in such activities while impaired. Compared to the reversible MAOI antidepressant drug moclobemide, more impairment of vigilance occurs with trazodone.[39]
Heart
Case reports have noted cardiac arrhythmias emerging in relation to trazodone treatment, both in patients with pre-existing mitral valve prolapse and in patients with negative personal and family histories of cardiac disease.[40]
QT prolongation has been reported with trazodone therapy. Arrhythmia identified include isolated PVCs, ventricular couplets, and in two patients short episodes (three to four beats) of ventricular tachycardia. Several post-marketing reports have been made of arrhythmia in trazodone-treated patients who have pre-existing cardiac disease and in some patients who did not have pre-existing cardiac disease. Until the results of prospective studies are available, patients with pre-existing cardiac disease should be closely monitored, particularly for cardiac arrhythmias. Trazodone is not recommended for use during the initial recovery phase of myocardial infarction. Concomitant administration of drugs that prolong the QT interval or that are inhibitors of CYP3A4 may increase the risk of cardiac arrhythmia.[41][42]
Priapism
A relatively rare side effect associated with trazodone is priapism, likely due to its antagonism at α-adrenergic receptors.[43] More than 200 cases have been reported, and the manufacturer estimated that the incidence of any abnormal erectile function is about one in 6,000 male patients treated with trazodone. The risk for this side effect appears to be greatest during the first month of treatment at low dosages (i.e. <150 mg/day). Early recognition of any abnormal erectile function is important, including prolonged or inappropriate erections, and should prompt discontinuation of trazodone treatment. Clinical reports have also described trazodone-associated psychosexual side effects in women, including increased libido, priapism of the clitoris, and spontaneous orgasms.[40][44]
Other
Rare cases of liver toxicity have been observed, possibly due to the formation of reactive metabolites.[45]
Elevated prolactin concentrations have been observed in people taking trazodone.[46]
Pregnancy and lactation
Sufficient data in humans are lacking. Use should be justified by the severity of the condition to be treated.[47][48]
Overdose
There are reported cases of high doses of trazodone precipitating serotonin syndrome.[49] There are also reports of patients taking multiple SSRIs with trazodone and precipitating serotonin syndrome.[49]
Trazodone appears to be relatively safer than TCAs, MAOIs, and a few of the other second-generation antidepressants in overdose situations, especially when it is the only agent taken. Fatalities are rare, and uneventful recoveries have been reported after ingestion of doses as high as 6,000–9,200 mg. In one report, 9 of 294 cases of overdose were fatal, and all nine patients had also taken other central nervous system (CNS) depressants. When trazodone overdoses occur, clinicians should carefully monitor for low blood pressure, a potentially serious toxic effect. In a report of a fatal trazodone overdose, torsades de pointes and complete atrioventricular block developed, along with subsequent multiple organ failure, with a trazodone plasma concentration of 25.4 mg/L on admission.[23][50][51][52]
There is no specific antidote for trazodone. Management of overdosage should, therefore, be symptomatic and supportive. Any person suspected of having taken an overdosage should be evaluated at a hospital as soon as possible. Activated charcoal, and forced diuresis may be useful in facilitating elimination of the drug, gastric lavage has been shown to not be useful unless done during the first hour after intake.[citation needed]
Interactions
Trazodone is metabolized by CYP3A4, a liver enzyme.[53] Inhibition of this enzyme by various other substances may delay its degradation, leading to high blood levels of trazodone. CYP3A4 may be inhibited by many other medications, herbs, and foods, and as such, trazodone may interact with these substances.
Pharmacology
Pharmacodynamics
| Site | Trazodone | mCPP | Species | Ref |
|---|---|---|---|---|
| SERT | 160–>10,000[55] | 202–432 | Human | [54][56][57] |
| NET | ≥8,500 | ≥1,940 | Human | [57][56] |
| DAT | ≥7,400 | ND | Human | [57][54] |
| 5-HT1A | 96–118 | 44–400 | Human | [54][58][59] |
| 5-HT1B | >10,000 | 89–501 | Human | [54][60] |
| 5-HT1D | 106 | 210–1,300 | Human | [54][59][61] |
| 5-HT1E | >10,000 | ND | Human | [54] |
| 5-HT1F | ND | ND | ND | ND |
| 5-HT2A | 20–45 | 32–398 | Human | [54][62][63][64] |
| 5-HT2B | 74–189 | 3.2–63 | Human | [54][62][65][66] |
| 5-HT2C | 224–402 | 3.4–251 | Human | [62][67][68][64] |
| 5-HT3 | >10,000 | 427 | Human | [54] |
| 5-HT4 | ND | ND | ND | ND |
| 5-HT5A | >10,000 | 1,354 | Human | [54] |
| 5-HT6 | >10,000 | 1,748 | Human | [54] |
| 5-HT7 | 1,782 | 163 | Human | [54] |
| α1 | 12–42 | 97–2,900 | Human | [56][58][59][69] |
| α1A | 153 | 1,386 | Human | [54] |
| α1B | ND | 915 | Human | [54] |
| α1D | ND | ND | ND | ND |
| α2 | 106–490 | 112–570 | Human | [58][56][59][69] |
| α2A | 728 | 145 | Human | [54] |
| α2B | ND | 106 | Human | [54] |
| α2C | 155 | 124 | Human | [54] |
| β | >10,000 | 2,500 | Human | [54][59] |
| β1 | >10,000 | 2,359 | Human | [54] |
| β2 | >10,000 | 3,474 | Human | [54] |
| D1 | 3,730 | 7,000 | Human | [54][59] |
| D2 | ≥3,500 | >10,000 | Human | [54][58][70][59] |
| D3 | 353 | >10,000 | Rat | [54][59] |
| D4 | 703 | ND | Human | [54] |
| D5 | >10,000 | >10,000 | Human | [54][59] |
| H1 | 220–1,100 | 326 | Human | [54][69][58] |
| H2 | 3,290 | ND | Human | [54] |
| H3 | >10,000 | ND | Guinea pig | [54] |
| H4 | >10,000 | ND | Human | [54] |
| mAChRs | >10,000 | >10,000 | Human | [54][70][58][59] |
| nAChRs | >10,000 | >10,000 | Human | [54] |
| σ1 | >10,000 | ND | Rat | [54] |
| σ2 | 536 | 8,350 | Rat | [54] |
| I1 | ND | 759 | Rat | [54] |
| NMDAR (MK-801) |
>10,000 | ND | Rat | [54] |
| VDCCs | >10,000 | 6,043 | Rat | [54] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||||
Trazodone is generally described as acting as a potent serotonin 5-HT2A and α1-adrenergic receptor antagonist, a weak serotonin reuptake inhibitor (SRI), and a weak antihistamine or histamine H1 receptor inverse agonist.[71] Its 5-HT2A receptor antagonism and weak serotonin reuptake inhibition form the basis of its common label as a serotonin antagonist and reuptake inhibitor (SARI).[72] Trazodone, both itself and via its major active metabolite meta-chlorophenylpiperazine (mCPP),[73] also binds to a variety of other receptors.[54] It is an antagonist at most or all of the receptors it binds to except the 5-HT1A receptor, where it acts as a partial agonist similarly to buspirone and tandospirone but with comparatively greater intrinsic activity.[54][74][75] Conversely, mCPP is a non-selective agonist of most of the serotonin receptors it binds to.[76] A range of weak affinities (Ki) have been reported for trazodone at the human histamine H1 receptor including 220 nM,[54] 350 nM,[69] 500 nM,[77] and 1,100 nM.[58]
Correspondence to clinical effects
Trazodone acts predominantly as a 5-HT2A receptor antagonist to mediate its therapeutic benefits against anxiety and depression.[78] Its inhibitory effects on serotonin reuptake and 5-HT2C receptors are comparatively weak.[78] Hence, trazodone does not have similar properties to selective serotonin reuptake inhibitors (SSRIs)[78] and is not particularly associated with increased appetite and weight gain, unlike other 5-HT2C antagonists like mirtazapine.[79][80] Moderate 5-HT1A partial agonism is likely to contribute to trazodone's antidepressant and anxiolytic actions to some extent as well.[74][75][81]
Roughly half of brain 5-HT2A receptors are blocked by 1 mg of trazodone and essentially all 5-HT2A receptors are saturated at 10 mg of trazodone, but the clinically effective hypnotic doses of trazodone are in the 25–100 mg range.[82][83]
The combined actions of 5-HT2A and 5HT2C receptor antagonism with serotonin reuptake inhibition only occur at moderate to high doses of trazodone.[84] Doses of trazodone lower than those effective for antidepressant action are frequently used for the effective treatment of insomnia.[84] Low doses exploit trazodone's potent actions as a 5-HT2A receptor antagonist, and its properties as an antagonist of H1 and α1-adrenergic receptors, but do not adequately exploit its SERT or 5-HT2C inhibition properties, which are weaker.[84] Since insomnia is one of the most frequent residual symptoms of depression after treatment with an SSRI, a hypnotic is often necessary for patients with a major depressive episode.[84] Not only can a hypnotic potentially relieve the insomnia itself, but treating insomnia in patients with major depression may also increase remission rates due to improvement of other symptoms such as loss of energy and depressed mood.[84] Thus, the ability of low doses of trazodone to improve sleep in depressed patients may be an important mechanism whereby trazodone can augment the efficacy of other antidepressants.[84]
Trazodone's potent α1-adrenergic blockade may cause some side effects like orthostatic hypotension and sedation.[85] Conversely, along with 5-HT2A and H1 receptor antagonism, it may contribute to its efficacy as a hypnotic. Trazodone lacks any affinity for the muscarinic acetylcholine receptors, so does not produce anticholinergic side effects.
mCPP, a non-selective serotonin receptor modulator and serotonin releasing agent, is the major active metabolite of trazodone and has been suggested to possibly play a role in its therapeutic benefits.[86][87][88] However, scientific research has not supported this hypothesis, and mCPP may actually antagonize trazodone's efficacy as well as produce additional side effects.[89][90][91][92][93]
Pharmacokinetics
Trazodone is well absorbed after oral administration, with mean peak blood levels obtained at about one hour after ingestion. Absorption is somewhat delayed and enhanced by food. The drug is 89–95% protein-bound.[94] Trazodone is extensively metabolized by the liver, with three or four major metabolites having been identified in the human body, particularly mCPP,[53] which may contribute to the side effect profile of trazodone and which probably accounts for trazodone's serotonergic effects.[95] Levels of trazodone are about 10-fold those of mCPP with treatment.[96] The mean blood elimination half-life of trazodone is biphasic: the first phase's half-life is 3 to 6 hours, and the following phase's half-life is 5 to 9 hours. Metabolites are conjugated to gluconic acid or glutathione and around 70 to 75% of 14C-labelled trazodone was found to be excreted in the urine within 72 hours.[97] The remaining drug and its metabolites are excreted in the faeces via biliary elimination. Less than 1% of the drug is excreted in its unchanged form.[94]
As a consequence of the production of mCPP as a metabolite, patients administered trazodone may test positive on EMIT II urine tests for the presence of MDMA ("ecstasy").[98]
Chemistry
Trazodone is a triazolopyridine derivative and a phenylpiperazine that is chemically related to nefazodone and etoperidone, each of which are derivatives of it.[99][100][101]
History
Trazodone was developed in Italy, in the 1960s, by Angelini Research Laboratories as a second-generation antidepressant.[102][103] It was developed according to the mental pain hypothesis, which was postulated from studying patients and which proposes that major depression is associated with a decreased pain threshold.[104] In sharp contrast to most other antidepressants available at the time of its development, trazodone showed minimal effects on muscarinic cholinergic receptors. Trazodone was patented and marketed in many countries all over the world. It was approved by the Food and Drug Administration (FDA) in 1981[105] and was the first non-tricyclic antidepressant approved in the US.[106]
Society and culture
Cost
The cost in the United Kingdom for the NHS is about £7.46 per month as of 2019.[16] In the United States, the wholesale cost is about US$4.53 per month as of 2018.[17] In 2017, it was the 30th most commonly prescribed medication in the United States, with more than 22 million prescriptions.[18][19]
-
Trazodone costs (US)
-
Trazodone prescriptions (US)
Generic names
Trazodone is the generic name of the drug and its INN, BAN, and DCF, while trazodone hydrochloride is its USAN, USP, BANM, and JAN.[107][108][109][110]
Brand names
Trazodone has been marketed under a large number of brand names throughout the world.[108][110] Major brand names include Desyrel (worldwide), Donaren (Brazil), Molipaxin (Ireland, United Kingdom), Oleptro (United States), Trazorel (Canada), and Trittico (worldwide).[108][110]
References
- ↑ 1.0 1.1 "Trazodone". Drugs.com. Archived from the original on 16 September 2018. Retrieved 9 February 2019.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ "MicroMedex DrugPoints - Trazodone". Pharmacy Choice. Archived from the original on 20 April 2017. Retrieved 20 April 2017.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ 3.0 3.1 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 21 January 2021. Retrieved 9 September 2020.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ 4.0 4.1 Hubbard, John R.; Martin, Peter R. (2001). Substance Abuse in the Mentally and Physically Disabled. CRC Press. p. 26. ISBN 9780824744977. Archived from the original on 1 August 2020. Retrieved 6 August 2020.
- ↑ 5.0 5.1 5.2 5.3 5.4 Truven Health Analytics, Inc. DrugPoint System (Internet) [cited 2013 Oct 1]. Greenwood Village, CO: Thomsen Healthcare; 2013.[failed verification]
- ↑ "Trazodone". DrugBank. Archived from the original on 12 July 2017. Retrieved 7 June 2015.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Lemke, Thomas L.; Williams, David A. (2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. p. 615. ISBN 9781609133450. Archived from the original on 17 September 2018. Retrieved 6 August 2020.
- ↑ Sheldon H. Preskorn; Christina Y. Stanga; John P. Feighner; Ruth Ross (6 December 2012). Antidepressants: Past, Present and Future. Springer Science & Business Media. pp. 68–. ISBN 978-3-642-18500-7.
- ↑ Schatzberg AF, Nemeroff CB (2017). The American Psychiatric Association Publishing Textbook of Psychopharmacology, Fifth Edition. American Psychiatric Pub. pp. 460–. ISBN 978-1-58562-523-9.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 "Trazodone Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 19 September 2018. Retrieved 8 January 2018.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ 11.0 11.1 11.2 Ton, Joey (15 November 2021). "#302 Still awake? Trazodone for insomnia". CFPCLearn. Archived from the original on 28 March 2023. Retrieved 14 June 2023.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ "Trazodone Use During Pregnancy". Drugs.com. Archived from the original on 10 August 2019. Retrieved 7 January 2018.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Stahl, Stephen M. (2008). Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Cambridge University Press. p. 567. ISBN 9780521857024. Archived from the original on 11 August 2019. Retrieved 6 August 2020.
{{cite book}}: More than one of|accessdate=and|access-date=specified (help); More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Lemke, Thomas L.; Williams, David A. (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. p. 586. ISBN 9780781768795. Archived from the original on 12 August 2019. Retrieved 6 August 2020.
- ↑ British national formulary: BNF 69 (69th ed.). British Medical Association. 2015. pp. 257–258. ISBN 9780857111562.
- ↑ 16.0 16.1 British national formulary: BNF 76 (76th ed.). Pharmaceutical Press. 2018. p. 367. ISBN 9780857113382.
- ↑ 17.0 17.1 "NADAC as of 2018-01-03". Centers for Medicare and Medicaid Services. Archived from the original on 24 June 2019. Retrieved 7 January 2018.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ 18.0 18.1 "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 11 April 2020.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ 19.0 19.1 "Trazodone Hydrochloride - Drug Usage Statistics". ClinCalc. Archived from the original on 11 April 2020. Retrieved 11 April 2020.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ "Desyrel – FDA Prescribing Information". Drugs.com. Archived from the original on 6 June 2015. Retrieved 4 June 2015.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ British National Formulary (BNF) 65. London, UK: Pharmaceutical Press. 2013. p. 247. ISBN 9780857110848.
- ↑ Nierenberg AA, Adler LA, Peselow E, Zornberg G, Rosenthal M (July 1994). "Trazodone for antidepressant-associated insomnia". Am J Psychiatry. 151 (7): 1069–72. doi:10.1176/ajp.151.7.1069. PMID 8010365.
- ↑ 23.0 23.1 23.2 23.3 Schatzberg, AF; Nemeroff, CB, eds. (2009). Textbook of Psychopharmacology (4th ed.). Washington, D.C.: American Psychiatric Publishing. ISBN 978-1-58562-309-9.
- ↑ "Understanding the Pharmacologic Therapy for Complex Regional Pain Syndrome: Pharmacologic Therapy". Medscape.com. Archived from the original on 8 March 2021. Retrieved 14 March 2014.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Prasad A (February 1985). "Efficacy of trazodone as an anti obsessional agent". Pharmacol. Biochem. Behav. 22 (2): 347–8. doi:10.1016/0091-3057(85)90403-4. PMID 3983224.
- ↑ Roccatagliata G; Albano C; Maffini M; Farelli S (1980). "Alcohol withdrawal syndrome: treatment with trazodone". Int Pharmacopsychiatry. 15 (2): 105–10. doi:10.1159/000468420. PMID 6108298.
- ↑ Le Bon O, Murphy JR, Staner L, Hoffmann G, Kormoss N, Kentos M, Dupont P, Lion K, Pelc I, Verbanck P (August 2003). "Double-blind, placebo-controlled study of the efficacy of trazodone in alcohol post-withdrawal syndrome: polysomnographic and clinical evaluations". J Clin Psychopharmacol. 23 (4): 377–83. doi:10.1097/01.jcp.0000085411.08426.d3. PMID 12920414.
- ↑ Borras L, de Timary P, Constant EL, Huguelet P, Eytan A (November 2006). "Successful treatment of alcohol withdrawal with trazodone". Pharmacopsychiatry. 39 (6): 232. doi:10.1055/s-2006-951385. PMID 17124647.
- ↑ Singh SP, Singh V, Kar N, Chan K (September 2010). "Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis". Br J Psychiatry. 197 (3): 174–9. doi:10.1192/bjp.bp.109.067710. PMID 20807960.
- ↑ Fink HA, MacDonald R, Rutks IR, Wilt TJ (September 2003). "Trazodone for erectile dysfunction: a systematic review and meta-analysis". BJU Int. 92 (4): 441–6. doi:10.1046/j.1464-410X.2003.04358.x. PMID 12930437. Archived from the original on 29 August 2021. Retrieved 6 August 2020.
{{cite journal}}: More than one of|accessdate=and|access-date=specified (help); More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ "Trazodone - FDA prescribing information, side effects and uses". Archived from the original on 8 July 2020. Retrieved 6 August 2020.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ "Desyrel (Trazodone Hydrochloride): Side Effects, Interactions, Warning, Dosage & Uses". Archived from the original on 2 September 2020. Retrieved 6 August 2020.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ "Oleptro™ (trazodone hydrochloride) extended-release tablets". Pharmacy and Therapeutics. 36 (2): 2–18. 2011. ISSN 1052-1372. PMC 3059557. PMID 21431085.
- ↑ "Trazodone (Oral Route) Proper Use - Mayo Clinic". www.mayoclinic.org. Archived from the original on 30 October 2020. Retrieved 11 February 2020.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Malhi GS (November 2015). "Antidepressants in bipolar depression: yes, no, maybe?". Evid Based Ment Health. 18 (4): 100–2. doi:10.1136/eb-2015-102229. PMID 26459471.
- ↑ "Webmd.com". Webmd.com. Archived from the original on 6 August 2014. Retrieved 14 March 2014.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Warner CH, Bobo W, Warner C, Reid S, Rachal J (August 2006). "Antidepressant discontinuation syndrome". Am Fam Physician. 74 (3): 449–56. PMID 16913164.
- ↑ "FDA - Trazodone Prescribing Information" (PDF). Archived (PDF) from the original on 14 July 2020.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Wesnes KA, Simpson PM, Christmas L, Anand R, McClelland GR (1989). "The effects of moclobemide on cognition". J. Neural Transm. Suppl. 28: 91–102. PMID 2677245.
- ↑ 40.0 40.1 Schatzberg, AF; Nemeroff, CB, eds. (2009). Textbook of Psychopharmacology (4th ed.). Washington, D.C.: American Psychiatric Publishing. ISBN 978-1-58562-309-9.
- ↑ "Trazodone PRODUCT MONOGRAPH" (PDF). 2015. Archived (PDF) from the original on 8 March 2021. Retrieved 6 August 2020.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ "HIGHLIGHTS OF PRESCRIBING INFORMATION" (PDF). U.S. Food and Drug Administration. 2017. Archived (PDF) from the original on 14 July 2020. Retrieved 6 August 2020.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Abber JC, Lue TF, Luo JA, Juenemann KP, Tanagho EA (May 1987). "Priapism induced by chlorpromazine and trazodone: mechanism of action". J. Urol. 137 (5): 1039–42. doi:10.1016/s0022-5347(17)44355-2. PMID 3573170.
- ↑ Battaglia C, Venturoli S (October 2009). "Persistent genital arousal disorder and trazodone. Morphometric and vascular modifications of the clitoris. A case report". J Sex Med. 6 (10): 2896–900. doi:10.1111/j.1743-6109.2009.01418.x. PMID 19674253.
- ↑ Kalgutkar AS, Henne KR, Lame ME, Vaz AD, Collin C, Soglia JR, Zhao SX, Hop CE (June 2005). "Metabolic activation of the nontricyclic antidepressant trazodone to electrophilic quinone-imine and epoxide intermediates in human liver microsomes and recombinant P4503A4". Chem. Biol. Interact. 155 (1–2): 10–20. doi:10.1016/j.cbi.2005.03.036. PMID 15978881.
- ↑ Otani K, Yasui N, Kaneko S, Ishida M, Ohkubo T, Osanai T, Sugawara K, Fukushima Y (June 1995). "Trazodone treatment increases plasma prolactin concentrations in depressed patients". Int Clin Psychopharmacol. 10 (2): 115–7. doi:10.1097/00004850-199506000-00009. PMID 7673654.
- ↑ Einarson A, Bonari L, Voyer-Lavigne S, Addis A, Matsui D, Johnson Y, Koren G (March 2003). "A multicentre prospective controlled study to determine the safety of trazodone and nefazodone use during pregnancy". Can J Psychiatry. 48 (2): 106–10. doi:10.1177/070674370304800207. PMID 12655908.
- ↑ Verbeeck RK, Ross SG, McKenna EA (September 1986). "Excretion of trazodone in breast milk". Br J Clin Pharmacol. 22 (3): 367–70. doi:10.1111/j.1365-2125.1986.tb02903.x. PMC 1401139. PMID 3768252.
- ↑ 49.0 49.1 Cushing TA (24 April 2018). "Selective Serotonin Reuptake Inhibitor Toxicity". Medscape. WebMD LLC. Archived from the original on 10 July 2020. Retrieved 22 December 2018.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Martínez MA, Ballesteros S, Sánchez de la Torre C, Almarza E (2005). "Investigation of a fatality due to trazodone poisoning: case report and literature review". J Anal Toxicol. 29 (4): 262–8. doi:10.1093/jat/29.4.262. PMID 15975258.
- ↑ de Meester A, Carbutti G, Gabriel L, Jacques JM (2001). "Fatal overdose with trazodone: case report and literature review". Acta Clin Belg. 56 (4): 258–61. doi:10.1179/acb.2001.038. PMID 11603256.
- ↑ Rakel RE (1987). "The greater safety of trazodone over tricyclic antidepressant agents: 5-year experience in the United States". Psychopathology. 20 (Suppl 1): 57–63. doi:10.1159/000284524. PMID 3321131.
- ↑ 53.0 53.1 Rotzinger S, Fang J, Baker GB (June 1998). "Trazodone is metabolized to m-chlorophenylpiperazine by CYP3A4 from human sources". Drug Metab. Dispos. 26 (6): 572–5. PMID 9616194.
- ↑ 54.00 54.01 54.02 54.03 54.04 54.05 54.06 54.07 54.08 54.09 54.10 54.11 54.12 54.13 54.14 54.15 54.16 54.17 54.18 54.19 54.20 54.21 54.22 54.23 54.24 54.25 54.26 54.27 54.28 54.29 54.30 54.31 54.32 54.33 54.34 54.35 54.36 54.37 54.38 54.39 Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 8 March 2021. Retrieved 14 August 2017.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 25 May 2018.
- ↑ 56.0 56.1 56.2 56.3 Owens MJ, Morgan WN, Plott SJ, Nemeroff CB (1997). "Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites". J. Pharmacol. Exp. Ther. 283 (3): 1305–22. PMID 9400006.
- ↑ 57.0 57.1 57.2 Tatsumi M, Groshan K, Blakely RD, Richelson E (1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". Eur. J. Pharmacol. 340 (2–3): 249–58. doi:10.1016/s0014-2999(97)01393-9. PMID 9537821.
- ↑ 58.0 58.1 58.2 58.3 58.4 58.5 58.6 Cusack B, Nelson A, Richelson E (1994). "Binding of antidepressants to human brain receptors: focus on newer generation compounds". Psychopharmacology. 114 (4): 559–65. doi:10.1007/bf02244985. PMID 7855217.
- ↑ 59.0 59.1 59.2 59.3 59.4 59.5 59.6 59.7 59.8 59.9 Hamik A, Peroutka SJ (1989). "1-(m-chlorophenyl)piperazine (mCPP) interactions with neurotransmitter receptors in the human brain". Biol. Psychiatry. 25 (5): 569–75. doi:10.1016/0006-3223(89)90217-5. PMID 2537663.
- ↑ Boess FG, Martin IL (1994). "Molecular biology of 5-HT receptors". Neuropharmacology. 33 (3–4): 275–317. doi:10.1016/0028-3908(94)90059-0. PMID 7984267.
- ↑ Hamblin MW, Metcalf MA (1991). "Primary structure and functional characterization of a human 5-HT1D-type serotonin receptor". Mol. Pharmacol. 40 (2): 143–8. PMID 1652050.
- ↑ 62.0 62.1 62.2 Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M (2004). "Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors". Naunyn Schmiedebergs Arch. Pharmacol. 370 (2): 114–23. doi:10.1007/s00210-004-0951-4. PMID 15322733.
- ↑ Bonhaus DW, Bach C, DeSouza A, Salazar FH, Matsuoka BD, Zuppan P, Chan HW, Eglen RM (1995). "The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors". Br. J. Pharmacol. 115 (4): 622–8. doi:10.1111/j.1476-5381.1995.tb14977.x. PMC 1908489. PMID 7582481.
- ↑ 64.0 64.1 Rothman RB, Baumann MH (2009). "Serotonergic drugs and valvular heart disease". Expert Opin Drug Saf. 8 (3): 317–29. doi:10.1517/14740330902931524. PMC 2695569. PMID 19505264.
- ↑ Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL (2000). "Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications". Circulation. 102 (23): 2836–41. doi:10.1161/01.cir.102.23.2836. PMID 11104741.
- ↑ Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ (1999). "Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells". Br. J. Pharmacol. 128 (1): 13–20. doi:10.1038/sj.bjp.0702751. PMC 1571597. PMID 10498829.
- ↑ Bentley JM, Adams DR, Bebbington D, Benwell KR, Bickerdike MJ, Davidson JE, Dawson CE, Dourish CT, Duncton MA, Gaur S, George AR, Giles PR, Hamlyn RJ, Kennett GA, Knight AR, Malcolm CS, Mansell HL, Misra A, Monck NJ, Pratt RM, Quirk K, Roffey JR, Vickers SP, Cliffe IA (2004). "Indoline derivatives as 5-HT(2C) receptor agonists". Bioorg. Med. Chem. Lett. 14 (9): 2367–70. doi:10.1016/j.bmcl.2003.05.001. PMID 15081042.
- ↑ Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM (1997). "RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist". Neuropharmacology. 36 (4–5): 621–9. doi:10.1016/s0028-3908(97)00049-x. PMID 9225287.
- ↑ 69.0 69.1 69.2 69.3 Richelson E, Nelson A (1984). "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro". J. Pharmacol. Exp. Ther. 230 (1): 94–102. PMID 6086881.
- ↑ 70.0 70.1 Stanton T, Bolden-Watson C, Cusack B, Richelson E (1993). "Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics". Biochem. Pharmacol. 45 (11): 2352–4. doi:10.1016/0006-2952(93)90211-e. PMID 8100134.
- ↑ Fagiolini A, Comandini A, Catena Dell'Osso M, Kasper S (2012). "Rediscovering trazodone for the treatment of major depressive disorder". CNS Drugs. 26 (12): 1033–49. doi:10.1007/s40263-012-0010-5. PMC 3693429. PMID 23192413.
- ↑ Khouzam HR (2017). "A review of trazodone use in psychiatric and medical conditions". Postgrad Med. 129 (1): 140–148. doi:10.1080/00325481.2017.1249265. PMID 27744763.
- ↑ Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 586–. ISBN 978-0-7817-6879-5. Archived from the original on 29 August 2021. Retrieved 6 August 2020.
- ↑ 74.0 74.1 Raffa RB, Shank RP, Vaught JL (1992). "Etoperidone, trazodone and MCPP: in vitro and in vivo identification of serotonin 5-HT1A (antagonistic) activity". Psychopharmacology. 108 (3): 320–6. doi:10.1007/BF02245118. PMID 1387963.
- ↑ 75.0 75.1 Odagaki Y, Toyoshima R, Yamauchi T (May 2005). "Trazodone and its active metabolite m-chlorophenylpiperazine as partial agonists at 5-HT1A receptors assessed by [35S]GTPgammaS binding". J. Psychopharmacol. (Oxford). 19 (3): 235–41. doi:10.1177/0269881105051526. PMID 15888508.
- ↑ Kahn RS, Wetzler S (1991). "m-Chlorophenylpiperazine as a probe of serotonin function". Biol. Psychiatry. 30 (11): 1139–66. doi:10.1016/0006-3223(91)90184-n. PMID 1663792.
- ↑ Krystal AD, Richelson E, Roth T (2013). "Review of the histamine system and the clinical effects of H1 antagonists: basis for a new model for understanding the effects of insomnia medications". Sleep Med Rev. 17 (4): 263–72. doi:10.1016/j.smrv.2012.08.001. PMID 23357028.
- ↑ 78.0 78.1 78.2 Marek GJ, McDougle CJ, Price LH, Seiden LS (1992). "A comparison of trazodone and fluoxetine: implications for a serotonergic mechanism of antidepressant action". Psychopharmacology. 109 (1–2): 2–11. doi:10.1007/BF02245475. PMID 1365657.
- ↑ Vanina Y, Podolskaya A, Sedky K, Shahab H, Siddiqui A, Munshi F, Lippmann S (July 2002). "Body weight changes associated with psychopharmacology". Psychiatr Serv. 53 (7): 842–7. doi:10.1176/appi.ps.53.7.842. PMID 12096167.
- ↑ Watanabe N, Omori IM, Nakagawa A, Cipriani A, Barbui C, McGuire H, et al. (January 2010). "Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression: systematic review and meta-analysis". CNS Drugs. 24 (1): 35–53. doi:10.2165/11319480-000000000-00000. PMID 20030418.
- ↑ Kinney GG, Griffith JC, Hudzik TJ (July 1998). "Antidepressant-like effects of 5-hydroxytryptamine1A receptor agonists on operant responding under a response duration differentiation schedule". Behav Pharmacol. 9 (4): 309–18. doi:10.1097/00008877-199807000-00002. PMID 10065919.
- ↑ Jaffer, Karim Yahia; Chang, Tiffany; Vanle, Brigitte; Dang, Jonathan; Steiner, Alexander J.; Loera, Natalie; Abdelmesseh, Marina; Danovitch, Itai; Ishak, Waguih William (1 August 2017). "Trazodone for Insomnia: A Systematic Review". Innovations in Clinical Neuroscience. 14 (7–8): 24–34. ISSN 2158-8333. PMC 5842888. PMID 29552421.
- ↑ Stahl, SM (October 2009). "Mechanism of action of trazodone: a multifunctional drug". CNS Spectrums. 14 (10): 536–46. doi:10.1017/s1092852900024020. PMID 20095366.
- ↑ 84.0 84.1 84.2 84.3 84.4 84.5 Stahl, S.M. (2013). Stahl's Essential Psychopharmacology (4th ed.). Cambridge University Press. ISBN 978-1107686465.
- ↑ Asayesh K (December 1986). "Combination of trazodone and phenothiazines: a possible additive hypotensive effect". Canadian Journal of Psychiatry. 31 (9): 857–8. doi:10.1177/070674378603100913. PMID 3802006.
- ↑ Melzacka M; Rurak; Vetulani (1980). "Preliminary study of the biotransformation of two new drugs, trazodone and etoperidone". Polish Journal of Pharmacology and Pharmacy. 32 (4): 551–6. PMID 7255270.
- ↑ Fong MH, Garattini S, Caccia S (October 1982). "1-m-Chlorophenylpiperazine is an active metabolite common to the psychotropic drugs trazodone, etoperidone and mepiprazole". J. Pharm. Pharmacol. 34 (10): 674–5. doi:10.1111/j.2042-7158.1982.tb04701.x. PMID 6128394.
- ↑ Maes M, Westenberg H, Vandoolaeghe E, Demedts P, Wauters A, Neels H, Meltzer HY (October 1997). "Effects of trazodone and fluoxetine in the treatment of major depression: therapeutic pharmacokinetic and pharmacodynamic interactions through formation of meta-chlorophenylpiperazine". J Clin Psychopharmacol. 17 (5): 358–64. doi:10.1097/00004714-199710000-00004. PMID 9315986.
- ↑ Mihara K, Yasui-Furukori N, Kondo T, et al. (August 2002). "Relationship between plasma concentrations of trazodone and its active metabolite, m-chlorophenylpiperazine, and its clinical effect in depressed patients". Therapeutic Drug Monitoring. 24 (4): 563–6. doi:10.1097/00007691-200208000-00016. PMID 12142643.
- ↑ Li AA, Marek GJ, Hand TH, Seiden LS (February 1990). "Antidepressant-like effects of trazodone on a behavioral screen are mediated by trazodone, not the metabolite m-chlorophenylpiperazine". Eur. J. Pharmacol. 177 (3): 137–44. doi:10.1016/0014-2999(90)90263-6. PMID 2311675.
- ↑ Vetulani J, Sansone M, Baran L, Hano J (1984). "Opposite action of m-chlorophenylpiperazine on avoidance depression induced by trazodone and pimozide in CD-1 mice". Psychopharmacology. 83 (2): 166–8. doi:10.1007/BF00429728. PMID 6431467.
- ↑ Kast RE (2009). "Trazodone generates m-CPP: in 2008 risks from m-CPP might outweigh benefits of trazodone". World Journal of Biological Psychiatry. 10 (4 Pt 2): 682–5. doi:10.1080/15622970902836022. PMID 19384678.
- ↑ Workman EA, Tellian F, Short D (May 1992). "Trazodone induction of migraine headache through mCPP". Am J Psychiatry. 149 (5): 712b–712. doi:10.1176/ajp.149.5.712b. PMID 1575270.
- ↑ 94.0 94.1 "Trazodone". www.drugbank.ca. Archived from the original on 12 July 2017. Retrieved 31 January 2019.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Garattini, S (1985). "Active drug metabolites. An overview of their relevance in clinical pharmacokinetics". Clinical Pharmacokinetics. 10 (3): 216–27. doi:10.2165/00003088-198510030-00002. PMID 2861928.
- ↑ Roy J. Vaz; Thomas Klabunde (9 April 2008). Antitargets: Prediction and Prevention of Drug Side Effects. John Wiley & Sons. pp. 149–. ISBN 978-3-527-62147-7. Archived from the original on 29 August 2021. Retrieved 6 August 2020.
{{cite book}}: More than one of|accessdate=and|access-date=specified (help); More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Jauch R, Kopitar Z, Prox A, Zimmer A (1976). "[Pharmacokinetics and metabolism of trazodone in man (author's transl)]". Arzneimittelforschung (in German). 26 (11): 2084–9. PMID 1037253.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ Logan BK, Costantino AG, Rieders EF, Sanders D (November 2010). "Trazodone, meta-chlorophenylpiperazine (an hallucinogenic drug and trazodone metabolite), and the hallucinogen trifluoromethylphenylpiperazine cross-react with the EMIT®II ecstasy immunoassay in urine". J Anal Toxicol. 34 (9): 587–9. doi:10.1093/jat/34.9.587. PMID 21073812.
- ↑ Akritopoulou-Zanze, Irini (2012). "6. Arylpiperazine-Based 5-HT1A Receptor Partial Agonists and 5-HT2A Antagonists for the Treatment of Autism, Depression, Anxiety, Psychosis, and Schizophrenia". In Dinges, Jürgen; Lamberth, Clemens (eds.). Bioactive heterocyclic compound classes pharmaceuticals. Weinheim: Wiley-VCH. ISBN 9783527664450.
- ↑ Dörwald, Florencioa Zaragoza, ed. (2012). "46. Arylalkylamines". Lead optimization for medicinal chemists : pharmacokinetic properties of functional groups and organic compounds. Weinheim: Wiley-VCH. ISBN 9783527645640.
- ↑ Haria M, Fitton A, McTavish D (April 1994). "Trazodone. A review of its pharmacology, therapeutic use in depression and therapeutic potential in other disorders". Drugs Aging. 4 (4): 331–55. doi:10.2165/00002512-199404040-00006. PMID 8019056.
- ↑ Gorecki, Dennis K.J.; Verbeeck, Roger K. (1987). "Trazondone Hydrochloride". In Forey, Klaus (ed.). Profiles of Drug Substances, Excipients and Related Methodology Vol. 16. Academic Press. p. 695. ISBN 9780080861111.
- ↑ Wegener, Gregers (30 March 2016). "Ban & Silvestrini's Trazodone". inhn.org. Archived from the original on 20 March 2017. Retrieved 4 June 2017.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Silvestrini B (1989). "Trazodone: from the mental pain to the "dys-stress" hypothesis of depression". Clin Neuropharmacol. 12 (Suppl 1): S4–10. doi:10.1097/00002826-198901001-00002. PMID 2568177.
- ↑ "Trazodone: Common sleep drug is little-known antidepressant - Consumer Reports". Consumer Reports. August 2015. Archived from the original on 12 October 2017. Retrieved 6 August 2020.
{{cite news}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Eisen, Michael S.; Taylor, Duncan B.; Riblet, Leslie A. (2012). "Atypical Psychotropic Agents". In Williams, Michael; Malick, Jeffrey B. (eds.). Drug Discovery and Development. Springer Science & Business Media. p. 388. ISBN 9781461248286. Archived from the original on 29 August 2021. Retrieved 6 August 2020.
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. ISBN 978-1-4757-2085-3. Archived from the original on 3 August 2020. Retrieved 6 August 2020.
{{cite book}}: More than one of|accessdate=and|access-date=specified (help); More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ 108.0 108.1 108.2 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 1050–1052. ISBN 978-3-88763-075-1. Archived from the original on 29 August 2021. Retrieved 6 August 2020.
- ↑ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 279–. ISBN 978-94-011-4439-1. Archived from the original on 16 July 2020. Retrieved 6 August 2020.
{{cite book}}: More than one of|accessdate=and|access-date=specified (help); More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ 110.0 110.1 110.2 "Trazodone". Drugs.com. Archived from the original on 16 September 2018. Retrieved 6 August 2020.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help)
External links
| External sites: | |
|---|---|
| Identifiers: |
|
- Pages using duplicate arguments in template calls
- CS1 errors: redundant parameter
- All articles with failed verification
- Articles with failed verification from April 2017
- Articles with invalid date parameter in template
- CS1 maint: unrecognized language
- Use dmy dates from May 2011
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from February 2016
- 5-HT1A agonists
- 5-HT2A antagonists
- Alpha-1 blockers
- Antidepressants
- Anxiolytics
- H1 receptor antagonists
- Hypnotics
- 2-(3-(4-(3-chlorophenyl)piperazin-1-yl)propyl)-1,2,4-triazol-3-ones
- Serotonin reuptake inhibitors
- Triazolopyridines
- Ureas
- RTT


