Hyperhomocysteinemia
| Hyperhomocysteinemia | |
|---|---|
| Other names: Hyperhomocysteinaemia | |
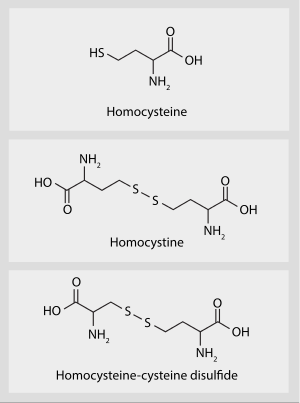 | |
| Total plasma homocysteine | |
Hyperhomocysteinemia is a medical condition characterized by an abnormally high level of total homocysteine (that is, including homocystine and homocysteine-cysteine disulfide) in the blood, conventionally described as above 15 μmol/L.[1]
As a consequence of the biochemical reactions in which homocysteine is involved, deficiencies of vitamin B6, folic acid (vitamin B9), and vitamin B12 can lead to high homocysteine levels.[2] Other possible causes of hyperhomocysteinemia include genetics, excessive methionine intake, and other diseases. [3]
Hyperhomocysteinemia is typically managed with vitamin B6, vitamin B9 and vitamin B12 supplementation.[4] Hyperhomocysteinemia is a risk factor for cardiovascular disease; however, supplements of these vitamins may not improve cardiovascular disease outcomes.[5]
Signs and symptoms
Elevated levels of homocysteine have been associated with a number of disease states.
Cardiovascular risks
Elevated homocysteine is a known risk factor for cardiovascular disease as well as thrombosis.[6] It has also been shown to be associated with microalbuminuria which is a strong indicator of the risk of future cardiovascular disease and renal dysfunction.[7] Homocysteine degrades and inhibits the formation of the three main structural components of arteries: collagen, elastin and proteoglycans. In proteins, homocysteine permanently degrades cysteine disulfide bridges and lysine amino acid residues,[8] affecting structure and function.
Neuropsychiatric illness
Evidence exists linking elevated homocysteine levels with vascular dementia[9] and Alzheimer's disease.[10][11][12] There is also evidence that elevated homocysteine levels and low levels of vitamin B6 and B12 are risk factors for mild cognitive impairment and dementia.[13] Oxidative stress induced by homocysteine may also play a role in schizophrenia.[14]
Bone health
Elevated levels of homocysteine have also been linked to increased fractures in elderly persons. Homocysteine auto-oxidizes and reacts with reactive oxygen intermediates, damaging endothelial cells and increasing the risk of thrombus formation.[15][16]
Ectopia lentis
Homocystinuria is the second most common cause of heritable ectopia lentis. Homocystinuria is an autosomal recessive metabolic disorder most often caused by a near absence of cystathionine b-synthetase. It is associated with intellectual disability, osteoporosis, chest deformities, and increased risk of thrombotic episodes. Lens dislocation occurs in 90% of patients, and is thought to be due to decreased zonular integrity due to the enzymatic defect. Lens dislocation in homocystinuria is usually bilateral and in 60% of cases occurs in the inferior or nasal direction.[citation needed]
Causes
Vitamin deficiency
Deficiencies of vitamins B6, B9 and B12 can lead to high homocysteine levels.[2] Vitamin B12 acts as a cofactor for the enzyme methionine synthase (which forms part of the S-adenosylmethionine (SAM) biosynthesis and regeneration cycle). Vitamin B12 deficiency prevents the 5-methyltetrahydrofolate (5-MTHF) form of folate from being converted into THF due to the "methyl trap".[17] This disrupts the folate pathway and leads to an increase in homocysteine which damages cells (for example, damage to endothelial cells can result in increased risk of thrombosis).
Alcohol
Chronic consumption of alcohol may also result in increased plasma levels of homocysteine.[18][19]
Tobacco
Smokeless tobacco is implicated as risk factor for hyperhomocysteinemia.[20] Smoking also causes hyperhomocysteinemia[21]
Genetic

Homocysteine is a non-protein amino acid, synthesized from methionine and either recycled back into methionine or converted into cysteine with the aid of the B-group vitamins.
- About 50% of homocysteine[citation needed] is converted back to methionine by remethylation via the methionine synthase major pathway. This requires active folate and vitamin B12, in order to donate a methyl group. Active folate is known as 5-methyltetrahydrofolate (5-MTHF).
- Another pathway for the conversion of homocysteine back to methionine also exists, involving methylation with trimethylglycine (also called betaine or abbreviated to TMG) as a methyl donor.
- The remaining homocysteine is transsulfurated to cysteine, with vitamin B6 as the co-factor.
Genetic defects in 5-MTHF reductase can consequently lead to hyperhomocysteinemia. The most common polymorphisms are known as MTHFR C677T and MTR A2756G.[23][24] These polymorphisms occur in about 10% of the world's population.[citation needed] Elevations of homocysteine can also occur in the rare hereditary disease homocystinuria.
Diagnosis
A blood test can be performed to quantify total homocysteine concentration in the plasma, of which approximately 80% is generally protein-bound. Classification of hyperhomocysteinemia is defined with respect to serum concentration as follows:[citation needed]
- Moderate: 15–30 nmol/mL (or μmol/L)
- Intermediate: 30–100 nmol/mL
- Severe: > 100 nmol/mL
If total homocysteine concentration is not found to be elevated, but clinical suspicion is still high, an oral methionine loading challenge several hours prior to quantification of homocysteine concentration may be used to increased sensitivity for marginal abnormalities of homocysteine metabolism.[25]
Fasting for 10 hours is sometimes recommended prior to measurement of homocysteine levels, but this may not be necessary for diagnostic yield.[26]
Treatment
Vitamins B6, B9, or B12 supplements (alone or combined), while they lower homocysteine level, do not change the risk of heart disease or prevent death in people who have heart disease when compared to standard care or to an inactive supplement in a clinical trial.[27] When combined with medicine to reduce blood pressure (antihypertensive drugs), it is not clear if treatments that lower homocysteine can help prevent a stroke in some people.[27] Hypotheses have been offered to address the failure of homocysteine-lowering therapies to reduce cardiovascular events. When folic acid is given as a supplement, it may increase the build-up of arterial plaque. A second hypothesis involves the methylation of genes in vascular cells by folic acid and vitamin B12, which may also accelerate plaque growth. Finally, altered methylation may catalyse l-arginine to asymmetric dimethylarginine, which is known to increase the risk of vascular disease.[28]
See also
References
- ↑ Guo, H; Chi, J; Xing, Y; Wang, P (2009). "Influence of folic acid on plasma homocysteine levels & arterial endothelial function in patients with unstable angina". The Indian Journal of Medical Research. 129 (3): 279–84. PMID 19491420.
- ↑ 2.0 2.1 Miller, J. W.; Nadeau, M. R.; Smith, D; Selhub, J (1994). "Vitamin B-6 deficiency vs folate deficiency: Comparison of responses to methionine loading in rats". The American Journal of Clinical Nutrition. 59 (5): 1033–9. doi:10.1093/ajcn/59.5.1033. PMID 8172087.
- ↑ Kim J, Kim H, Roh H, Kwon Y (2018). "Causes of hyperhomocysteinemia and its pathological significance". Arch Pharm Res. 41 (4): 372–383. doi:10.1007/s12272-018-1016-4. PMID 29552692. Archived from the original on 2023-07-18. Retrieved 2023-05-31.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Stehouwer, Coen DA; Guldener, Coen van (2005). "Homocysteine-lowering treatment: An overview". Expert Opinion on Pharmacotherapy. 2 (9): 1449–60. doi:10.1517/14656566.2.9.1449. PMID 11585023. S2CID 45945199.
- ↑ Martí-Carvajal, Arturo J.; Solà, Ivan; Lathyris, Dimitrios (15 January 2015). Martí-Carvajal, Arturo J (ed.). "Homocysteine-lowering interventions for preventing cardiovascular events". The Cochrane Database of Systematic Reviews. 1: CD006612. doi:10.1002/14651858.CD006612.pub4. ISSN 1469-493X. PMC 4164174. PMID 25590290.
- ↑ Cattaneo, Marco (1999). "Hyperhomocysteinemia, atherosclerosis and thrombosis". Thrombosis and Haemostasis. 81 (2): 165–76. doi:10.1055/s-0037-1614438. PMID 10063987. S2CID 13228673. Archived from the original on 2018-07-21. Retrieved 2016-12-27.
- ↑ Jager, A.; Kostense, P. J.; Nijpels, G.; Dekker, J. M.; Heine, R. J.; Bouter, L. M.; Donker, A. J. M.; Stehouwer, C. D. A. (2001). "Serum Homocysteine Levels Are Associated with the Development of (Micro)albuminuria : The Hoorn Study". Arteriosclerosis, Thrombosis, and Vascular Biology. 21 (1): 74–81. doi:10.1161/01.ATV.21.1.74. PMID 11145936.
- ↑ Jakubowski, H (2006). "Pathophysiological consequences of homocysteine excess". The Journal of Nutrition. 136 (6 Suppl): 1741S–1749S. doi:10.1093/jn/136.6.1741S. PMID 16702349.
- ↑ McVeigh, Catherine; Passmore, Peter (September 2006). "Vascular dementia: prevention and treatment". Clinical Interventions in Aging. 1 (3): 229–235. doi:10.2147/ciia.2006.1.3.229. ISSN 1176-9092. PMC 2695177. PMID 18046875.
- ↑ Morris, Martha Savaria (2003). "Homocysteine and Alzheimer's disease". The Lancet Neurology. 2 (7): 425–8. doi:10.1016/s1474-4422(03)00438-1. PMID 12849121. S2CID 20443022.
- ↑ Smach, Mohamed Ali; Jacob, Nelly; Golmard, Jean-Louis; Charfeddine, Bassem; Lammouchi, Turkia; Ben Othman, Leila; Dridi, Hedi; Bennamou, Soufien; Limem, Khalifa (2011). "Folate and Homocysteine in the Cerebrospinal Fluid of Patients with Alzheimer's Disease or Dementia: A Case Control Study". European Neurology. 65 (5): 270–8. doi:10.1159/000326301. PMID 21474939. S2CID 7689901.
- ↑ Smith, A. David; Smith, Stephen M.; De Jager, Celeste A.; Whitbread, Philippa; Johnston, Carole; Agacinski, Grzegorz; Oulhaj, Abderrahim; Bradley, Kevin M.; Jacoby, Robin; Refsum, Helga (2010). "Homocysteine-Lowering by B Vitamins Slows the Rate of Accelerated Brain Atrophy in Mild Cognitive Impairment: A Randomized Controlled Trial". PLOS ONE. 5 (9): e12244. Bibcode:2010PLoSO...512244S. doi:10.1371/journal.pone.0012244. PMC 2935890. PMID 20838622.
- ↑ Stanger, Olaf; Fowler, Brian; Piertzik, Klaus; Huemer, Martina; Haschke-Becher, Elisabeth; Semmler, Alexander; Lorenzl, Stefan; Linnebank, Michael (2014). "Homocysteine, folate and vitamin B12in neuropsychiatric diseases: Review and treatment recommendations" (PDF). Expert Review of Neurotherapeutics. 9 (9): 1393–412. doi:10.1586/ern.09.75. PMID 19769453. S2CID 13246020. Archived (PDF) from the original on 2020-10-24. Retrieved 2023-05-31.
- ↑ Dietrich-Muszalska, Anna; Malinowska, Joanna; Olas, Beata; Głowacki, Rafal; Bald, Edward; Wachowicz, Barbara; Rabe-Jabłońska, Jolanta (2012). "The Oxidative Stress May be Induced by the Elevated Homocysteine in Schizophrenic Patients". Neurochemical Research. 37 (5): 1057–62. doi:10.1007/s11064-012-0707-3. PMC 3321271. PMID 22270909.
- ↑ McLean, Robert R.; Jacques, Paul F.; Selhub, Jacob; Tucker, Katherine L.; Samelson, Elizabeth J.; Broe, Kerry E.; Hannan, Marian T.; Cupples, L. Adrienne; Kiel, Douglas P. (2004). "Homocysteine as a Predictive Factor for Hip Fracture in Older Persons". New England Journal of Medicine. 350 (20): 2042–9. doi:10.1056/NEJMoa032739. PMID 15141042. S2CID 22853996. Archived from the original on 2021-04-17. Retrieved 2023-05-31.
- ↑ Van Meurs, Joyce B.J.; Dhonukshe-Rutten, Rosalie A.M.; Pluijm, Saskia M.F.; Van Der Klift, Marjolein; De Jonge, Robert; Lindemans, Jan; De Groot, Lisette C.P.G.M.; Hofman, Albert; Witteman, Jacqueline C.M.; Van Leeuwen, Johannes P.T.M.; Breteler, Monique M.B.; Lips, Paul; Pols, Huibert A.P.; Uitterlinden, André G. (2004). "Homocysteine Levels and the Risk of Osteoporotic Fracture". New England Journal of Medicine. 350 (20): 2033–41. doi:10.1056/NEJMoa032546. hdl:1765/8452. PMID 15141041. Archived from the original on 2020-10-19. Retrieved 2023-05-31.
- ↑ Nijhout, H. Frederik; Reed, Michael C.; Budu, Paula; Ulrich, Cornelia M. (2004). "A Mathematical Model of the Folate Cycle: new insights into folate homeostasis". Journal of Biological Chemistry. 279 (53): 55008–16. doi:10.1074/jbc.M410818200. PMID 15496403.
- ↑ Bleich, S.; Bleich, K; Kropp, S; Bittermann, H. J.; Degner, D; Sperling, W; Rüther, E; Kornhuber, J (2001). "Moderate alcohol consumption in social drinkers raises plasma homocysteine levels: A contradiction to the 'French Paradox'?". Alcohol and Alcoholism. 36 (3): 189–92. doi:10.1093/alcalc/36.3.189. PMID 11373253.
- ↑ Bleich, Stefan; Carl, Marco; Bayerlein, Kristina; Reulbach, Udo; Biermann, Teresa; Hillemacher, Thomas; b??Nsch, Dominikus; Kornhuber, Johannes (2005). "Evidence of Increased Homocysteine Levels in Alcoholism: The Franconian Alcoholism Research Studies (FARS)". Alcoholism: Clinical & Experimental Research. 29 (3): 334–6. doi:10.1097/01.alc.0000156083.91214.59. PMID 15770107.
- ↑ Iqbal MP, Yakub M (2013). "Smokeless tobacco use: a risk factor for hyperhomocysteinemia in a Pakistani population". PLOS ONE. 8 (12): e83826. Bibcode:2013PLoSO...883826I. doi:10.1371/journal.pone.0083826. PMC 3871626. PMID 24376761.
- ↑ Haj Mouhamed D, Ezzaher A, Neffati F, Douki W, Najjar MF (March 2011). "Effect of cigarette smoking on plasma homocysteine concentrations". Clinical Chemistry and Laboratory Medicine. 49 (3): 479–83. doi:10.1515/CCLM.2011.062. PMID 21143017. S2CID 34110392.
- ↑ Zuhra, Karim; Augsburger, Fiona; Majtan, Tomas; Szabo, Csaba (30 April 2020). "Cystathionine-β-Synthase: Molecular Regulation and Pharmacological Inhibition". Biomolecules. 10 (5): 697. doi:10.3390/biom10050697. ISSN 2218-273X. Retrieved 17 July 2023.
- ↑ Qin, Xianhui; Li, Jianping; Cui, Yimin; Liu, Zeyuan; Zhao, Zhigang; Ge, Junbo; Guan, Deming; Hu, Jian; Wang, Yanni; Zhang, Fumin; Xu, Xin; Wang, Xiaobin; Xu, Xiping; Huo, Yong (2012). "MTHFR C677T and MTR A2756G polymorphisms and the homocysteine lowering efficacy of different doses of folic acid in hypertensive Chinese adults". Nutrition Journal. 11: 2. doi:10.1186/1475-2891-11-2. PMC 3274435. PMID 22230384.
- ↑ Yakub, Mohsin; Moti, Naushad; Parveen, Siddiqa; Chaudhry, Bushra; Azam, Iqbal; Iqbal, Mohammad Perwaiz (2012). "Polymorphisms in MTHFR, MS and CBS Genes and Homocysteine Levels in a Pakistani Population". PLOS ONE. 7 (3): e33222. Bibcode:2012PLoSO...733222Y. doi:10.1371/journal.pone.0033222. PMC 3310006. PMID 22470444.
- ↑ Kang, S. S.; Wong, P. W. (1996-01-26). "Genetic and nongenetic factors for moderate hyperhomocyst(e)inemia". Atherosclerosis. 119 (2): 135–138. doi:10.1016/0021-9150(95)05648-3. ISSN 0021-9150. PMID 8808490.
- ↑ Fokkema, M. Rebecca; Gilissen, Marleen F.; Doormaal, Jasper J. van; Volmer, Marcel; Kema, Ido P.; Muskiet, Frits A. J. (2003-05-01). "Fasting vs Nonfasting Plasma Homocysteine Concentrations for Diagnosis of Hyperhomocysteinemia". Clinical Chemistry. 49 (5): 818–821. doi:10.1373/49.5.818. ISSN 0009-9147. PMID 12709379.
- ↑ 27.0 27.1 Martí-Carvajal, Arturo J.; Solà, Ivan; Lathyris, Dimitrios; Dayer, Mark (2017). "Homocysteine-lowering interventions for preventing cardiovascular events". The Cochrane Database of Systematic Reviews. 8 (9): CD006612. doi:10.1002/14651858.CD006612.pub5. ISSN 1469-493X. PMC 6483699. PMID 28816346.
- ↑ Watson, KE (Fall 2006). "Lowering levels of lipids and homocysteine". Reviews in Cardiovascular Medicine. 7 (4): 248–50. PMID 17224870.
External links
| Classification | |
|---|---|
| External resources |
- Pages with script errors
- CS1 maint: multiple names: authors list
- All articles with unsourced statements
- Articles with unsourced statements from June 2022
- Articles with invalid date parameter in template
- Articles with unsourced statements from May 2014
- Articles with unsourced statements from June 2013
- Amino acid metabolism disorders
