Exemestane
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /ˌɛksəˈmɛˌsteɪn/ EK-sə-ME-stayn |
| Trade names | Aromasin |
| Other names | FCE-24304 |
| |
| Clinical data | |
| Drug class | Aromatase inhibitor; Antiestrogen |
| Main uses | Breast cancer[2] |
| Side effects | Hot flushes, tiredness, joint pain, trouble sleeping, increased sweating[3] |
| Pregnancy category |
|
| Routes of use | By mouth |
| Duration of action | 4–5 days[citation needed] |
| Typical dose | 25 mg OD[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607006 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | ~60%[citation needed] |
| Protein binding | 90% |
| Metabolism | Liver (CYP3A4, aldo-keto reductase) |
| Elimination half-life | 24 hours |
| Excretion | Urine and feces ~ 1:1 (mainly metabolites) |
| Chemical and physical data | |
| Formula | C20H24O2 |
| Molar mass | 296.410 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Exemestane, sold under the brand name Aromasin among others, is a medication used to treat breast cancer.[2] Specifically it is used for early estrogen receptor positive disease or advanced disease that has become resistant to tamoxifen.[3] It is taken by mouth.[2]
Common side effects include hot flushes, tiredness, joint pain, trouble sleeping, and increased sweating.[3] Other side effects may include osteoporosis.[3] Use in pregnancy may harm the baby.[3] It is a aromatase inhibitor which blocks the creation of estrogen.[3][4]
Exemestane was approved for medical use in the United Kingdom in 1998 and the United States in 1999.[5][6] It is available as a generic medication.[2] In the United Kingdom a month costs the NHS about £21.[2] This amount in the United States costs about 50 USD.[7]
Medical uses
Exemestane is indicated for the adjuvant treatment of postmenopausal women with estrogen-receptor positive early breast cancer who have received two to three years of tamoxifen and are switched to it for completion of a total of five consecutive years of adjuvant hormonal therapy.[8]
Exemestane is also indicated for the treatment of advanced breast cancer in postmenopausal women whose disease has progressed following tamoxifen therapy.[9]
For premenopausal women with hormone-receptor–positive breast cancer, adjuvant treatment with ovarian suppression plus the aromatase inhibitor exemestane, as compared with ovarian suppression plus tamoxifen, provides a new treatment option that reduces the risk of recurrence. The TEXT and SOFT trials demonstrated improved disease free survival in people treated with exemestane and ovarian suppression compared to the tamoxifen and ovarian suppression group. Premenopausal women who receive ovarian suppression may now benefit from an aromatase inhibitor, a class of drugs that until now has been recommended only for postmenopausal women.[10]
Dosage
It is used at a dose of 25 mg per day.[2]
Contraindications
The drug is contraindicated in premenopausal women, which of course includes pregnant and lactating women.[11]
Side effects
The most common side effects (more than 10% of patients) are hot flashes and sweating, which are typical of estrogen deficiency as caused by exemestane, and also insomnia, headache, and joint pain. Nausea and fatigue are mainly observed in patients with advanced breast cancer.[11][12]
An occasional decrease in lymphocytes has been observed in approximately 20% of patients receiving Aromasin, particularly in patients with pre-existing lymphopenia.[13]
Exemestane has androgenic properties similarly to formestane and can produce androgenic side effects such as acne and weight gain, although these are generally associated with supratherapeutic dosages of the drug.[14]
Overdose
Single doses of up to at least 32-fold (800 mg), as well as continuous therapy with 24-fold (600 mg) the usual daily dose are well tolerated. No life-threatening overdosing is known in humans, but only in animal studies with 2000- to 4000-fold doses (adjusted to body surface area).[11]
Interactions
Exemestane is metabolized by the liver enzyme CYP3A4. While the CYP3A4 inhibitor ketoconazole had no significant effect on exemestane levels in a clinical trial, the strong CYP3A4 inductor rifampicin significantly cut exemenstane levels about in half (AUC −54%, Cmax −41% for a single dose), potentially compromising its effectiveness. Other 3A4 inductors such as carbamazepine and St John's Wort are expected to have similar effects.[11][12] The clinical relevance of this effect has not been investigated.[15]
Estrogens probably reduce exemestane effectiveness:[12] It would usually be counter-productive to reduce the body's estrogen synthesis with exemestane and then substitute estrogen with pharmaceuticals.
Pharmacology
Some breast cancers require estrogen to grow. Those cancers have estrogen receptors (ERs), and are called ER-positive. They may also be called estrogen-responsive, hormonally-responsive, or hormone-receptor-positive. Aromatase is an enzyme that synthesizes estrogen. Aromatase inhibitors block the synthesis of estrogen. This lowers the estrogen level, and slows the growth of cancers.
Pharmacodynamics
Exemestane is an oral steroidal aromatase inhibitor that is used in ER-positive breast cancer in addition to surgery and/or radiation in post-menopausal women.
The main source of estrogen is the ovaries in premenopausal women, while in post-menopausal women most of the body's estrogen is produced via the conversion of androgens into estrogen by the aromatase enzyme in the peripheral tissues (i.e. adipose tissue like that of the breast) and a number of sites in the brain. Estrogen is produced locally via the actions of the aromatase enzyme in these peripheral tissues where it acts locally. Any circulating estrogen in post-menopausal women as well as men is the result of estrogen escaping local metabolism and entering the circulatory system.[16]
Exemestane is an irreversible, steroidal aromatase inactivator of type I, structurally related to the natural substrate 4-androstenedione. It acts as a false substrate for the aromatase enzyme, and is processed to an intermediate that binds irreversibly to the active site of the enzyme causing its inactivation, an effect also known as "suicide inhibition." By being structurally similar to enzyme targets, exemestane permanently binds to the enzymes, preventing them from converting androgen into estrogen.[11]
Type II aromatase inhibitors such as anastrozole and letrozole, by contrast, are not steroids and work by interfering with the aromatase's heme.[15]
A study conducted on young adult males found that the estrogen suppression rate for exemestane varied from 35% for estradiol (E2) to 70% for estrone (E1).[17]
| Generation | Medication | Dosage | % inhibitiona | Classb | IC50c |
|---|---|---|---|---|---|
| First | Testolactone | 250 mg 4x/day p.o. | ? | Type I | ? |
| 100 mg 3x/week i.m. | ? | ||||
| Rogletimide | 200 mg 2x/day p.o. 400 mg 2x/day p.o. 800 mg 2x/day p.o. |
50.6% 63.5% 73.8% |
Type II | ? | |
| Aminoglutethimide | 250 mg mg 4x/day p.o. | 90.6% | Type II | 4,500 nM | |
| Second | Formestane | 125 mg 1x/day p.o. 125 mg 2x/day p.o. 250 mg 1x/day p.o. |
72.3% 70.0% 57.3% |
Type I | 30 nM |
| 250 mg 1x/2 weeks i.m. 500 mg 1x/2 weeks i.m. 500 mg 1x/1 week i.m. |
84.8% 91.9% 92.5% | ||||
| Fadrozole | 1 mg 1x/day p.o. 2 mg 2x/day p.o. |
82.4% 92.6% |
Type II | ? | |
| Third | Exemestane | 25 mg 1x/day p.o. | 97.9% | Type I | 15 nM |
| Anastrozole | 1 mg 1x/day p.o. 10 mg 1x/day p.o. |
96.7–97.3% 98.1% |
Type II | 10 nM | |
| Letrozole | 0.5 mg 1x/day p.o. 2.5 mg 1x/day p.o. |
98.4% 98.9%–>99.1% |
Type II | 2.5 nM | |
| Footnotes: a = In postmenopausal women. b = Type I: Steroidal, irreversible (substrate-binding site). Type II: Nonsteroidal, reversible (binding to and interference with the cytochrome P450 heme moiety). c = In breast cancer homogenates. Sources: See template. | |||||
Pharmacokinetics
Exemestane is quickly absorbed from the gut, but undergoes a strong first-pass effect in the liver. Highest blood plasma concentrations are reached after 1.2 hours in breast cancer patients and after 2.9 hours in healthy subjects. Maximal aromatase inhibition occurs after two to three days.[15] 90% of the absorbed substance are bound to plasma proteins. The liver enzyme CYP3A4 oxidizes the methylidene group in position 6, and the 17-keto group (on the five-membered ring) is reduced by aldo-keto reductases to an alcohol. Of the resulting metabolites, 40% are excreted via the urine and 40% via the feces within a week. The original substance accounts for only 1% of excretion in the urine. The terminal half-life is 24 hours.[11][18]
Chemistry
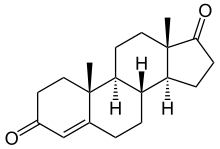
Exemestane is known chemically as 6-methylideneandrosta-1,4-diene-3,17-dione. Like the aromatase inhibitors formestane and atamestane, exemestane is a steroid that is structurally similar to 4-androstenedione, the natural substrate of aromatase. It is distinguished from the natural substance only by the methylidene group in position 6 and an additional double bond in position 1.[19]
Pure exemestane is a white to off-white powder that is soluble in DMSO to at least 20 mg/mL. Optical rotation [α]D is +250 to 300° (per g/100 cm³ and decimetre at 589 nm wavelength).[20]
History
US FDA approval was in October 1999.[21]
Society and culture
Performance enhancement
Exemestane has been used in doping to raise luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels, which in turn increases the ratio of male over female sexual hormones and so improves performance. The drug also counteracts gynecomastia as well as fat and water retention following excessive aromatase production due to testosterone doping.[22]
Along with other aromatase inhibitors, exemestane is on the World Anti-Doping Agency's list of prohibited substances.[23]
Research
Oral exemestane 25 mg/day for 2–3 years of adjuvant therapy was generally more effective than 5 years of continuous adjuvant tamoxifen in the treatment of postmenopausal women with early-stage estrogen receptor-positive/unknown receptor status breast in a large well-designed[citation needed] trial. Preliminary data from the open-label TEAM trial comparing exemestane with tamoxifen indicated in 2009 that exemestane 25 mg/day is also effective in the primary adjuvant treatment of early-stage breast cancer in postmenopausal women.[24]
Interim phase III trial results in 2011 showed that adding everolimus to exemestane therapy against advanced breast cancer can significantly improve progression-free survival compared with exemestane therapy alone.[25]
A Phase III trial was reported in 2011, concluding that the use of exemestane in postmenopausal women at an increased risk for breast cancer reduced the incidence of invasive breast cancer. In 4,560 women, after 35 months, the administration of exemestane at a dose of 25 mg/day resulted in a 65% reduction in the risk of breast cancer compared with placebo; annual incidence rates were 0.19% and 0.55%, respectively (hazard ratio: 0.35; 95% CI [0.18-0.70]; p = 0.002).[26]
References
- ↑ Exemestane Archived 2015-12-23 at the Wayback Machine at ChEBI
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 998. ISBN 978-0857114105.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "DailyMed - EXEMESTANE tablet". dailymed.nlm.nih.gov. Archived from the original on 21 March 2021. Retrieved 17 December 2021.
- ↑ "Exemestane". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 6 May 2021. Retrieved 17 December 2021.
- ↑ "Aromasin - Summary of Product Characteristics (SmPC) - (emc)". www.medicines.org.uk. Archived from the original on 13 May 2021. Retrieved 17 December 2021.
- ↑ "Exemestane Monograph for Professionals". Drugs.com. Archived from the original on 8 May 2021. Retrieved 17 December 2021.
- ↑ "Exemestane Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 21 October 2016. Retrieved 17 December 2021.
- ↑ Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, et al. (February 2007). "Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial". Lancet. 369 (9561): 559–70. doi:10.1016/S0140-6736(07)60200-1. PMID 17307102. S2CID 38977099.
- ↑ Aromasin For Advanced Breast Cancer Archived 2012-03-28 at the Wayback Machine on Aromasin.com
- ↑ Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Láng I, et al. (July 2014). "Adjuvant exemestane with ovarian suppression in premenopausal breast cancer". The New England Journal of Medicine. 371 (2): 107–18. doi:10.1056/NEJMoa1404037. PMC 4175521. PMID 24881463.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 Jasek, W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 656–660. ISBN 978-3-85200-181-4.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ 12.0 12.1 12.2 Drugs.com: Monograph on exemestane.
- ↑ "Aromasin - Summary of Product Characteristics (SMPC) - (Emc)". Archived from the original on 2021-05-08. Retrieved 2021-09-17.
- ↑ Buzdar AU, Robertson JF, Eiermann W, Nabholtz JM (November 2002). "An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrozole, letrozole, and exemestane". Cancer. 95 (9): 2006–16. doi:10.1002/cncr.10908. PMID 12404296. S2CID 34798824.
- ↑ 15.0 15.1 15.2 Dinnendahl V, Fricke U, eds. (2007). Arzneistoff-Profile (in German). Vol. 4 (21 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Simpson ER (September 2003). "Sources of estrogen and their importance". The Journal of Steroid Biochemistry and Molecular Biology. 86 (3–5): 225–30. doi:10.1016/S0960-0760(03)00360-1. PMID 14623515. S2CID 11210435.
- ↑ Mauras N, Lima J, Patel D, Rini A, di Salle E, Kwok A, Lippe B (December 2003). "Pharmacokinetics and dose finding of a potent aromatase inhibitor, aromasin (exemestane), in young males". The Journal of Clinical Endocrinology and Metabolism. 88 (12): 5951–6. doi:10.1210/jc.2003-031279. PMID 14671195.
- ↑ Mutschler E, Schäfer-Korting M (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 904. ISBN 3-8047-1763-2.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Steinhilber D, Schubert-Zsilavecz M, Roth HJ (2005). Medizinische Chemie (in German). Stuttgart: Deutscher Apotheker Verlag. pp. 467f. ISBN 3-7692-3483-9.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Sigma-Aldrich Co., Exemestane, ≥ 98% (HPLC).
- ↑ "Archive copy" (PDF). Archived (PDF) from the original on 2017-02-11. Retrieved 2021-09-17.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Sinner, D. (2007). Das Schwarze Buch – Anabole Steroide (in Deutsch). p. 133. ISBN 978-3-00-020944-4.
- ↑ Substance Classification Booklet Archived 2013-09-27 at the Wayback Machine, Canadian Centre for Ethics in Sport, Version 4.0, January 2009.
- ↑ Deeks ED, Scott LJ (2009). "Exemestane: a review of its use in postmenopausal women with breast cancer". Drugs. 69 (7): 889–918. doi:10.2165/00003495-200969070-00007. PMID 19441873. Archived from the original on 2011-10-08. Retrieved 2010-03-29.
- ↑ "Positive Trial Data Leads Novartis to Plan Breast Cancer Filing for Afinitor by Year End". 2011. Archived from the original on 2016-03-03. Retrieved 2021-09-17.
- ↑ Goss, Paul E. (June 6, 2011). "Exemestane Offers New Option for Breast Cancer Prevention". American Society of Clinical Oncology. Archived from the original on July 11, 2011. Retrieved June 6, 2011.
External links
| Identifiers: |
|
|---|
- Pages using duplicate arguments in template calls
- Webarchive template wayback links
- CS1 maint: unrecognized language
- CS1 maint: archived copy as title
- CS1 Deutsch-language sources (de)
- All articles with unsourced statements
- Articles with unsourced statements from November 2017
- Articles with invalid date parameter in template
- Articles with unsourced statements from December 2015
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Androgens and anabolic steroids
- Androstanes
- Aromatase inhibitors
- Diketones
- Enantiopure drugs
- Hormonal antineoplastic drugs
- Pfizer brands
- World Anti-Doping Agency prohibited substances
- RTT
