Tooth decay
| Tooth decay | |
|---|---|
| Other names: Dental caries, cavities, caries | |
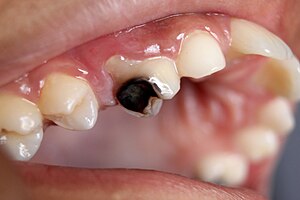 | |
| Destruction of a tooth by dental caries and disease. | |
| Pronunciation |
|
| Specialty | Dentistry |
| Symptoms | Pain, tooth loss, difficulty eating[1][2] |
| Complications | Inflammation around the tooth, tooth loss, infection or abscess formation[1][3] |
| Duration | Long term |
| Causes | Bacteria producing acid from food debris[4] |
| Risk factors | Diet high in simple sugar, diabetes mellitus, Sjögren syndrome, medications that decrease saliva[4] |
| Prevention | Low-sugar diet, teeth brushing, fluoride, flossing[2][5] |
| Medication | Paracetamol (acetaminophen), ibuprofen[6] |
| Frequency | 3.6 billion (2016)[7] |
Tooth decay, also known as dental caries or cavities, is a breakdown of teeth due to acids made by bacteria.[6] The cavities may be a number of different colors from yellow to black.[1] Symptoms may include pain and difficulty with eating.[1][2] Complications may include inflammation of the tissue around the tooth, tooth loss, and infection or abscess formation.[1][3]
The cause of cavities is acid from bacteria dissolving the hard tissues of the teeth (enamel, dentin and cementum).[4] The acid is produced by the bacteria when they break down food debris or sugar on the tooth surface.[4] Simple sugars in food are these bacteria's primary energy source and thus a diet high in simple sugar is a risk factor.[4] If mineral breakdown is greater than build up from sources such as saliva, caries results.[4] Risk factors include conditions that result in less saliva such as: diabetes mellitus, Sjögren syndrome and some medications.[4] Medications that decrease saliva production include antihistamines and antidepressants.[4] Dental caries are also associated with poverty, poor cleaning of the mouth, and receding gums resulting in exposure of the roots of the teeth.[6][8]
Prevention of dental caries includes regular cleaning of the teeth, a diet low in sugar, and small amounts of fluoride.[2][4][9] Brushing the teeth twice per day and flossing between the teeth once a day is recommended.[4][6] Fluoride may be acquired from water, salt or toothpaste among other sources.[2] Treating a mother's dental caries may decrease the risk in her children by decreasing the numbers of certain bacteria she may spread to them.[4] Screening can result in earlier detection.[6] Depending on the extent of destruction, various treatments can be used to restore the tooth to proper function or the tooth may be removed.[6] There is no known method to grow back large amounts of tooth.[10] The availability of treatment is often poor in the developing world.[2] Paracetamol (acetaminophen) or ibuprofen may be taken for pain.[6]
Worldwide, approximately 3.6 billion people (48% of the population) have dental caries in their permanent teeth as of 2016.[7] The World Health Organization estimates that nearly all adults have dental caries at some point in time.[2] In baby teeth it affects about 620 million people or 9% of the population.[11] They have become more common in both children and adults in recent years.[12] The disease is most common in the developed world due to greater simple sugar consumption and less common in the developing world.[6] Caries is Latin for "rottenness".[3]
Signs and symptoms
A person experiencing caries may not be aware of the disease.[13] The earliest sign of a new carious lesion is the appearance of a chalky white spot on the surface of the tooth, indicating an area of demineralization of enamel. This is referred to as a white spot lesion, an incipient carious lesion or a "microcavity".[14] As the lesion continues to demineralize, it can turn brown but will eventually turn into a cavitation ("cavity"). Before the cavity forms, the process is reversible, but once a cavity forms, the lost tooth structure cannot be regenerated. A lesion that appears dark brown and shiny suggests dental caries were once present but the demineralization process has stopped, leaving a stain. Active decay is lighter in color and dull in appearance.[15]
As the enamel and dentin are destroyed, the cavity becomes more noticeable. The affected areas of the tooth change color and become soft to the touch. Once the decay passes through enamel, the dentinal tubules, which have passages to the nerve of the tooth, become exposed, resulting in pain that can be transient, temporarily worsening with exposure to heat, cold, or sweet foods and drinks.[16] A tooth weakened by extensive internal decay can sometimes suddenly fracture under normal chewing forces. When the decay has progressed enough to allow the bacteria to overwhelm the pulp tissue in the center of the tooth, a toothache can result and the pain will become more constant. Death of the pulp tissue and infection are common consequences. The tooth will no longer be sensitive to hot or cold, but can be very tender to pressure.
Dental caries can also cause bad breath and foul tastes.[17] In highly progressed cases, an infection can spread from the tooth to the surrounding soft tissues. Complications such as cavernous sinus thrombosis and Ludwig angina can be life-threatening.[18][19][20]
Cause

Four things are required for caries formation: a tooth surface (enamel or dentin), caries-causing bacteria, fermentable carbohydrates (such as sucrose), and time.[21] This involves adherence of food to the teeth and acid creation by the bacteria that makes up the dental plaque.[22] However, these four criteria are not always enough to cause the disease and a sheltered environment promoting development of a cariogenic biofilm is required. The caries disease process does not have an inevitable outcome, and different individuals will be susceptible to different degrees depending on the shape of their teeth, oral hygiene habits, and the buffering capacity of their saliva. Dental caries can occur on any surface of a tooth that is exposed to the oral cavity, but not the structures that are retained within the bone.[23]
Tooth decay is caused by biofilm (dental plaque) lying on the teeth and maturing to become cariogenic (causing decay). Certain bacteria in the biofilm produce acid in the presence of fermentable carbohydrates such as sucrose, fructose, and glucose.[24][25]
Caries occur more often in people from the lower end of the socioeconomic scale than people from the upper end of the socioeconomic scale.[26]
Bacteria

The most common bacteria associated with dental cavities are the mutans streptococci, most prominently Streptococcus mutans and Streptococcus sobrinus, and lactobacilli. However, cariogenic bacteria (the ones that can cause the disease) are present in dental plaque, but they are usually in too low concentrations to cause problems unless there is a shift in the balance.[27] This is driven by local environmental change, such as frequent sugar intake or inadequate biofilm removal (toothbrushing).[28] If left untreated, the disease can lead to pain, tooth loss and infection.[29]
The mouth contains a wide variety of oral bacteria, but only a few specific species of bacteria are believed to cause dental caries: Streptococcus mutans and Lactobacillus species among them. Streptococcus mutans are gram-positive bacteria which constitute biofilms on the surface of teeth. These organisms can produce high levels of lactic acid following fermentation of dietary sugars and are resistant to the adverse effects of low pH, properties essential for cariogenic bacteria.[24] As the cementum of root surfaces is more easily demineralized than enamel surfaces, a wider variety of bacteria can cause root caries, including Lactobacillus acidophilus, Actinomyces spp., Nocardia spp., and Streptococcus mutans. Bacteria collect around the teeth and gums in a sticky, creamy-coloured mass called plaque, which serves as a biofilm. Some sites collect plaque more commonly than others, for example, sites with a low rate of salivary flow (molar fissures). Grooves on the occlusal surfaces of molar and premolar teeth provide microscopic retention sites for plaque bacteria, as do the interproximal sites. Plaque may also collect above or below the gingiva, where it is referred to as supra- or sub-gingival plaque, respectively.
These bacterial strains, most notably S. mutans, can be inherited by a child from a caretaker's kiss or through feeding premasticated.[30]
Dietary sugars
Bacteria in a person's mouth convert glucose, fructose, and most commonly sucrose (table sugar) into acids such as lactic acid through a glycolytic process called fermentation.[25] If left in contact with the tooth, these acids may cause demineralization, which is the dissolution of its mineral content. The process is dynamic, however, as remineralization can also occur if the acid is neutralized by saliva or mouthwash. Fluoride toothpaste or dental varnish may aid remineralization.[31] If demineralization continues over time, enough mineral content may be lost so that the soft organic material left behind disintegrates, forming a cavity or hole. The impact such sugars have on the progress of dental caries is called cariogenicity. Sucrose, although a bound glucose and fructose unit, is in fact more cariogenic than a mixture of equal parts of glucose and fructose. This is due to the bacteria utilising the energy in the saccharide bond between the glucose and fructose subunits. S.mutans adheres to the biofilm on the tooth by converting sucrose into an extremely adhesive substance called dextran polysaccharide by the enzyme dextransucranase.[32]
Exposure

The frequency with which teeth are exposed to cariogenic (acidic) environments affects the likelihood of caries development.[citation needed] After meals or snacks, the bacteria in the mouth metabolize sugar, resulting in an acidic by-product that decreases pH. As time progresses, the pH returns to normal due to the buffering capacity of saliva and the dissolved mineral content of tooth surfaces. During every exposure to the acidic environment, portions of the inorganic mineral content at the surface of teeth dissolve and can remain dissolved for two hours.[33] Since teeth are vulnerable during these acidic periods, the development of dental caries relies heavily on the frequency of acid exposure.
The carious process can begin within days of a tooth's erupting into the mouth if the diet is sufficiently rich in suitable carbohydrates. Evidence suggests that the introduction of fluoride treatments has slowed the process.[34] Proximal caries take an average of four years to pass through enamel in permanent teeth. Because the cementum enveloping the root surface is not nearly as durable as the enamel encasing the crown, root caries tend to progress much more rapidly than decay on other surfaces. The progression and loss of mineralization on the root surface is 2.5 times faster than caries in enamel. In very severe cases where oral hygiene is very poor and where the diet is very rich in fermentable carbohydrates, caries may cause cavities within months of tooth eruption. This can occur, for example, when children continuously drink sugary drinks from baby bottles (see later discussion).
Teeth
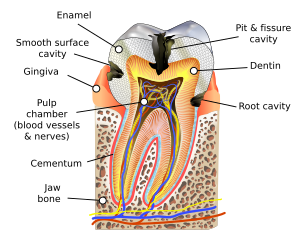
There are certain diseases and disorders affecting teeth that may leave an individual at a greater risk for cavities.
Molar incisor hypomineralization, which seems to be increasingly common.[35] While the cause is unknown it is thought to be a combination of genetic and environmental factors.[36] Possible contributing factors that have been investigated include systemic factors such as high levels of dioxins or polychlorinated biphenyl (PCB) in the mother's milk, premature birth and oxygen deprivation at birth, and certain disorders during the child's first 3 years such as such as mumps, diphtheria, scarlet fever, measles, hypoparathyroidism, malnutrition, malabsorption, hypovitaminosis D, chronic respiratory diseases, or undiagnosed and untreated coeliac disease, which usually presents with mild or absent gastrointestinal symptoms.[35][37][38][39][40][41]
Amelogenesis imperfecta, which occurs in between 1 in 718 and 1 in 14,000 individuals, is a disease in which the enamel does not fully form or forms in insufficient amounts and can fall off a tooth.[42] In both cases, teeth may be left more vulnerable to decay because the enamel is not able to protect the tooth.[43]
In most people, disorders or diseases affecting teeth are not the primary cause of dental caries. Approximately 96% of tooth enamel is composed of minerals.[44] These minerals, especially hydroxyapatite, will become soluble when exposed to acidic environments. Enamel begins to demineralize at a pH of 5.5.[45] Dentin and cementum are more susceptible to caries than enamel because they have lower mineral content.[46] Thus, when root surfaces of teeth are exposed from gingival recession or periodontal disease, caries can develop more readily. Even in a healthy oral environment, however, the tooth is susceptible to dental caries.
The evidence for linking malocclusion and/or crowding to dental caries is weak;[47][48] however, the anatomy of teeth may affect the likelihood of caries formation. Where the deep developmental grooves of teeth are more numerous and exaggerated, pit and fissure caries is more likely to develop (see next section). Also, caries is more likely to develop when food is trapped between teeth.
Other factors
Reduced salivary flow rate is associated with increased caries since the buffering capability of saliva is not present to counterbalance the acidic environment created by certain foods. As a result, medical conditions that reduce the amount of saliva produced by salivary glands, in particular the submandibular gland and parotid gland, are likely to lead to dry mouth and thus to widespread tooth decay. Examples include Sjögren syndrome, diabetes mellitus, diabetes insipidus, and sarcoidosis.[49] Medications, such as antihistamines and antidepressants, can also impair salivary flow. Stimulants, most notoriously methylamphetamine, also occlude the flow of saliva to an extreme degree. This is known as meth mouth. Tetrahydrocannabinol (THC), the active chemical substance in cannabis, also causes a nearly complete occlusion of salivation, known in colloquial terms as "cotton mouth". Moreover, 63% of the most commonly prescribed medications in the United States list dry mouth as a known side-effect.[49] Radiation therapy of the head and neck may also damage the cells in salivary glands, somewhat increasing the likelihood of caries formation.[50][51]
Susceptibility to caries can be related to altered metabolism in the tooth, in particular to fluid flow in the dentin. Experiments on rats have shown that a high-sucrose, cariogenic diet "significantly suppresses the rate of fluid motion" in dentin.[52]
The use of tobacco may also increase the risk for caries formation. Some brands of smokeless tobacco contain high sugar content, increasing susceptibility to caries.[53] Tobacco use is a significant risk factor for periodontal disease, which can cause the gingiva to recede.[54] As the gingiva loses attachment to the teeth due to gingival recession, the root surface becomes more visible in the mouth. If this occurs, root caries is a concern since the cementum covering the roots of teeth is more easily demineralized by acids than enamel.[55] Currently, there is not enough evidence to support a causal relationship between smoking and coronal caries, but evidence does suggest a relationship between smoking and root-surface caries.[56] Exposure of children to secondhand tobacco smoke is associated with tooth decay.[57]
Intrauterine and neonatal lead exposure promote tooth decay.[58][59][60][61][62][63][64] Besides lead, all atoms with electrical charge and ionic radius similar to bivalent calcium,[65] such as cadmium, mimic the calcium ion and therefore exposure to them may promote tooth decay.[66]
Poverty is also a significant social determinant for oral health.[67] Dental caries have been linked with lower socio-economic status and can be considered a disease of poverty.[68]
Forms are available for risk assessment for caries when treating dental cases; this system using the evidence-based Caries Management by Risk Assessment (CAMBRA).[69] It is still unknown if the identification of high-risk individuals can lead to more effective long-term patient management that prevents caries initiation and arrests or reverses the progression of lesions.[70]
Saliva also contains iodine and EGF. EGF results effective in cellular proliferation, differentiation and survival.[71] Salivary EGF, which seems also regulated by dietary inorganic iodine, plays an important physiological role in the maintenance of oral (and gastro-oesophageal) tissue integrity, and, on the other hand, iodine is effective in prevention of dental caries and oral health.[72]
Pathophysiology


Teeth are bathed in saliva and have a coating of bacteria on them (biofilm) that continually forms. The development of biofilm begins with pellicle formation. Pellicle is an acellular proteinaceous film which covers the teeth. Bacteria colonize on the teeth by adhering to the pellicle-coated surface. Over time, a mature biofilm is formed and this create a cariogenic environment on the tooth surface.[73][74] The minerals in the hard tissues of the teeth (enamel, dentin and cementum) are constantly undergoing processes of demineralization and remineralization. Dental caries results when the demineralization rate is faster than the remineralization and there is net mineral loss. This happens when there is an ecologic shift within the dental biofilm, from a balanced population of micro-organisms to a population that produce acids and can survive in an acid environment.[75]
Enamel
Tooth enamel is a highly mineralized acellular tissue, and caries act upon it through a chemical process brought on by the acidic environment produced by bacteria. As the bacteria consume the sugar and use it for their own energy, they produce lactic acid. The effects of this process include the demineralization of crystals in the enamel, caused by acids, over time until the bacteria physically penetrate the dentin. Enamel rods, which are the basic unit of the enamel structure, run perpendicularly from the surface of the tooth to the dentin. Since demineralization of enamel by caries, in general, follows the direction of the enamel rods, the different triangular patterns between pit and fissure and smooth-surface caries develop in the enamel because the orientation of enamel rods are different in the two areas of the tooth.[76]
As the enamel loses minerals, and dental caries progresses, the enamel develops several distinct zones, visible under a light microscope. From the deepest layer of the enamel to the enamel surface, the identified areas are the: translucent zone, dark zones, body of the lesion, and surface zone.[77] The translucent zone is the first visible sign of caries and coincides with a one to two percent loss of minerals.[78] A slight remineralization of enamel occurs in the dark zone, which serves as an example of how the development of dental caries is an active process with alternating changes.[79] The area of greatest demineralization and destruction is in the body of the lesion itself. The surface zone remains relatively mineralized and is present until the loss of tooth structure results in a cavitation.
Dentin
Unlike enamel, the dentin reacts to the progression of dental caries. After tooth formation, the ameloblasts, which produce enamel, are destroyed once enamel formation is complete and thus cannot later regenerate enamel after its destruction. On the other hand, dentin is produced continuously throughout life by odontoblasts, which reside at the border between the pulp and dentin. Since odontoblasts are present, a stimulus, such as caries, can trigger a biologic response. These defense mechanisms include the formation of sclerotic and tertiary dentin.[80]
In dentin from the deepest layer to the enamel, the distinct areas affected by caries are the advancing front, the zone of bacterial penetration, and the zone of destruction.[76] The advancing front represents a zone of demineralized dentin due to acid and has no bacteria present. The zones of bacterial penetration and destruction are the locations of invading bacteria and ultimately the decomposition of dentin. The zone of destruction has a more mixed bacterial population where proteolytic enzymes have destroyed the organic matrix. The innermost dentin caries has been reversibly attacked because the collagen matrix is not severely damaged, giving it potential for repair.

Sclerotic dentin
The structure of dentin is an arrangement of microscopic channels, called dentinal tubules, which radiate outward from the pulp chamber to the exterior cementum or enamel border.[81] The diameter of the dentinal tubules is largest near the pulp (about 2.5 μm) and smallest (about 900 nm) at the junction of dentin and enamel.[82] The carious process continues through the dentinal tubules, which are responsible for the triangular patterns resulting from the progression of caries deep into the tooth. The tubules also allow caries to progress faster.
In response, the fluid inside the tubules brings immunoglobulins from the immune system to fight the bacterial infection. At the same time, there is an increase of mineralization of the surrounding tubules.[83] This results in a constriction of the tubules, which is an attempt to slow the bacterial progression. In addition, as the acid from the bacteria demineralizes the hydroxyapatite crystals, calcium and phosphorus are released, allowing for the precipitation of more crystals which fall deeper into the dentinal tubule. These crystals form a barrier and slow the advancement of caries. After these protective responses, the dentin is considered sclerotic.
According to hydrodynamic theory, fluids within dentinal tubules are believed to be the mechanism by which pain receptors are triggered within the pulp of the tooth.[84] Since sclerotic dentin prevents the passage of such fluids, pain that would otherwise serve as a warning of the invading bacteria may not develop at first.
Tertiary dentin
In response to dental caries, there may be production of more dentin toward the direction of the pulp. This new dentin is referred to as tertiary dentin.[82] Tertiary dentin is produced to protect the pulp for as long as possible from the advancing bacteria. As more tertiary dentin is produced, the size of the pulp decreases. This type of dentin has been subdivided according to the presence or absence of the original odontoblasts.[85] If the odontoblasts survive long enough to react to the dental caries, then the dentin produced is called "reactionary" dentin. If the odontoblasts are killed, the dentin produced is called "reparative" dentin.
In the case of reparative dentin, other cells are needed to assume the role of the destroyed odontoblasts. Growth factors, especially TGF-β,[85] are thought to initiate the production of reparative dentin by fibroblasts and mesenchymal cells of the pulp.[86] Reparative dentin is produced at an average of 1.5 μm/day, but can be increased to 3.5 μm/day. The resulting dentin contains irregularly shaped dentinal tubules that may not line up with existing dentinal tubules. This diminishes the ability for dental caries to progress within the dentinal tubules.
Cementum
The incidence of cemental caries increases in older adults as gingival recession occurs from either trauma or periodontal disease. It is a chronic condition that forms a large, shallow lesion and slowly invades first the root's cementum and then dentin to cause a chronic infection of the pulp (see further discussion under classification by affected hard tissue). Because dental pain is a late finding, many lesions are not detected early, resulting in restorative challenges and increased tooth loss.[87]
Diagnosis

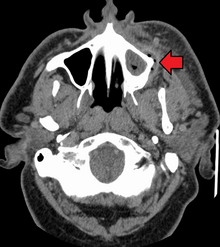
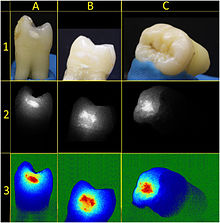
The presentation of caries is highly variable. However, the risk factors and stages of development are similar. Initially, it may appear as a small chalky area (smooth surface caries), which may eventually develop into a large cavitation. Sometimes caries may be directly visible. However other methods of detection such as X-rays are used for less visible areas of teeth and to judge the extent of destruction. Lasers for detecting caries allow detection without ionizing radiation and are now used for detection of interproximal decay (between the teeth).
Primary diagnosis involves inspection of all visible tooth surfaces using a good light source, dental mirror and explorer. Dental radiographs (X-rays) may show dental caries before it is otherwise visible, in particular caries between the teeth. Large areas of dental caries are often apparent to the naked eye, but smaller lesions can be difficult to identify. Visual and tactile inspection along with radiographs are employed frequently among dentists, in particular to diagnose pit and fissure caries.[89] Early, uncavitated caries is often diagnosed by blowing air across the suspect surface, which removes moisture and changes the optical properties of the unmineralized enamel.
Some dental researchers have cautioned against the use of dental explorers to find caries,[90] in particular sharp ended explorers. In cases where a small area of tooth has begun demineralizing but has not yet cavitated, the pressure from the dental explorer could cause a cavity. Since the carious process is reversible before a cavity is present, it may be possible to arrest caries with fluoride and remineralize the tooth surface. When a cavity is present, a restoration will be needed to replace the lost tooth structure.
At times, pit and fissure caries may be difficult to detect. Bacteria can penetrate the enamel to reach dentin, but then the outer surface may remineralize, especially if fluoride is present.[91] These caries, sometimes referred to as "hidden caries", will still be visible on X-ray radiographs, but visual examination of the tooth would show the enamel intact or minimally perforated.
The differential diagnosis for dental caries includes dental fluorosis and developmental defects of the tooth including hypomineralization of the tooth and hypoplasia of the tooth.[92]
The early carious lesion is characterized by demineralization of the tooth surface, altering the tooth's optical properties. Technology utilizing laser speckle image (LSI) techniques may provide a diagnostic aid to detect early carious lesions.[88]
Classification

Caries can be classified by location, etiology, rate of progression, and affected hard tissues.[93] These forms of classification can be used to characterize a particular case of tooth decay in order to more accurately represent the condition to others and also indicate the severity of tooth destruction. In some instances, caries is described in other ways that might indicate the cause. The G. V. Black classification is as follows:
- Class I – occlusal surfaces of posterior teeth, buccal or lingual pits on molars, lingual pit near cingulum of maxillary incisors
- Class II – proximal surfaces of posterior teeth
- Class III – interproximal surfaces of anterior teeth without incisal edge involvement
- Class IV – interproximal surfaces of anterior teeth with incisal edge involvement
- Class V – cervical third of facial or lingual surface of tooth
- Class VI – incisal or occlusal edge is worn away due to attrition
Early childhood caries
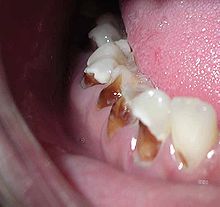
Early childhood caries (ECC), also known as "baby bottle caries," "baby bottle tooth decay" or "bottle rot," is a pattern of decay found in young children with their deciduous (baby) teeth. This must include the presence of at least one carious lesion on a primary tooth in a child under the age of 6 years.[94] The teeth most likely affected are the maxillary anterior teeth, but all teeth can be affected.[95] The name for this type of caries comes from the fact that the decay usually is a result of allowing children to fall asleep with sweetened liquids in their bottles or feeding children sweetened liquids multiple times during the day.[96]
Another pattern of decay is "rampant caries", which signifies advanced or severe decay on multiple surfaces of many teeth.[97] Rampant caries may be seen in individuals with xerostomia, poor oral hygiene, stimulant use (due to drug-induced dry mouth[98]), and/or large sugar intake. If rampant caries is a result of previous radiation to the head and neck, it may be described as radiation-induced caries. Problems can also be caused by the self-destruction of roots and whole tooth resorption when new teeth erupt or later from unknown causes.
Children at 6–12 months are at increased risk of developing dental caries. For other kids aged 12–18 months, dental caries develop on primary teeth and approximately twice yearly for permanent teeth.[99]
A range of studies have reported that there is a correlation between caries in primary teeth and caries in permanent teeth.[100][101]
Rate of progression
This section needs additional citations for verification. (November 2016) |
Temporal descriptions can be applied to caries to indicate the progression rate and previous history. "Acute" signifies a quickly developing condition, whereas "chronic" describes a condition that has taken an extended time to develop, in which thousands of meals and snacks, many causing some acid demineralization that is not remineralized, eventually result in cavities.
Recurrent caries, also described as secondary, are caries that appear at a location with a previous history of caries. This is frequently found on the margins of fillings and other dental restorations. On the other hand, incipient caries describes decay at a location that has not experienced previous decay. Arrested caries describes a lesion on a tooth that was previously demineralized but was remineralized before causing a cavitation. Fluoride treatment can help recalcification of tooth enamel as well as the use of amorphous calcium phosphate.
Micro-invasive interventions (such as dental sealant or resin infiltration) have been shown to slow down the progression of proximal decay.[102]
Affected hard tissue
Depending on which hard tissues are affected, it is possible to describe caries as involving enamel, dentin, or cementum. Early in its development, caries may affect only enamel. Once the extent of decay reaches the deeper layer of dentin, the term "dentinal caries" is used. Since cementum is the hard tissue that covers the roots of teeth, it is not often affected by decay unless the roots of teeth are exposed to the mouth. Although the term "cementum caries" may be used to describe the decay on roots of teeth, very rarely does caries affect the cementum alone.
Prevention

Oral hygiene
In the Western world, the primary approach to dental hygiene care consists of tooth-brushing and flossing. The purpose of oral hygiene is to remove and prevent the formation of plaque or dental biofilm,[103] although studies have shown this effect on caries is limited.[104] While there is no evidence that flossing prevents tooth decay,[105] the practice is still generally recommended.[5]
A toothbrush can be used to remove plaque on accessible surfaces, but not between teeth or inside pits and fissures on chewing surfaces. When used correctly, dental floss removes plaque from areas that could otherwise develop proximal caries but only if the depth of sulcus has not been compromised. Additional aids include interdental brushes, water picks, and mouthwashes. The use of rotational electric toothbrushes might reduce the risk of plaque and gingivitis, though it is unclear whether they are of clinical importance.[106]
However oral hygiene is effective at preventing gum disease (gingivitis / periodontal disease). Food is forced inside pits and fissures under chewing pressure, leading to carbohydrate-fuelled acid demineralisation where the brush, fluoride toothpaste, and saliva have no access to remove trapped food, neutralise acid, or remineralise tooth enamel. (Occlusal caries accounts for between 80 and 90% of caries in children (Weintraub, 2001).) Unlike brushing, fluoride leads to proven reduction in caries incidence by approximately 25%; higher concentrations of fluoride (>1,000 ppm) in toothpaste also helps prevents tooth decay, with the effect increasing with concentration up to a plateau.[107] A randomized clinical trial demonstrated that toothpastes that contain arginine have greater protection against tooth cavitation than the regular fluoride toothpastes containing 1450 ppm alone.[108][109] A Cochrane review has confirmed that the use of fluoride gels, normally applied by a dental professional from once to several times a year, assists in the prevention of tooth decay in children and adolescents, reiterating the importance of fluoride as the principal means of caries prevention.[110] Another review concluded that the supervised regular use of a fluoride mouthwash greatly reduced the onset of decay in the permanent teeth of children.[111]
Professional hygiene care consists of regular dental examinations and professional prophylaxis (cleaning). Sometimes, complete plaque removal is difficult, and a dentist or dental hygienist may be needed. Along with oral hygiene, radiographs may be taken at dental visits to detect possible dental caries development in high-risk areas of the mouth (e.g. "bitewing" X-rays which visualize the crowns of the back teeth).
Alternative methods of oral hygiene also exist around the world, such as the use of teeth cleaning twigs such as miswaks in some Middle Eastern and African cultures. There is some limited evidence demonstrating the efficacy of these alternative methods of oral hygiene.[112]
Dietary modification

People who eat more free sugars get more cavities, with cavities increasing exponentially with increasing sugar intake. Populations with less sugar intake have fewer cavities. In one population, in Nigeria, where sugar consumption was about 2g/day, only two percent of the population, of any age, had had a cavity.[113]
In the presence of sugar and other carbohydrates, bacteria in the mouth produce acids that can demineralize enamel, dentin, and cementum. The more frequently teeth are exposed to this environment, the more likely dental caries is to occur.[medical citation needed] Therefore, minimizing snacking is recommended, since snacking creates a continuous supply of nutrition for acid-creating bacteria in the mouth.[medical citation needed]
Chewy and sticky foods (such as candy, cookies, potato chips, and crackers) tend to adhere to teeth longer. However, dried fruits such as raisins and fresh fruit such as apples and bananas disappear from the mouth quickly, and do not appear to be a risk factor. Consumers are not good at guessing which foods stick around in the mouth.[114]
For children, the American Dental Association and the European Academy of Paediatric Dentistry recommend limiting the frequency of consumption of drinks with sugar, and not giving baby bottles to infants during sleep (see earlier discussion).[115][116] Parents are also recommended to avoid sharing utensils and cups with their infants to prevent transferring bacteria from the parent's mouth.[117]
It has been found that milk and certain kinds of cheese like cheddar cheese can help counter tooth decay if eaten soon after the consumption of foods potentially harmful to teeth.[citation needed]
Xylitol is a naturally occurring sugar alcohol that is used in different products as an alternative to sucrose (table sugar). As of 2015 the evidence concerning the use of xylitol in chewing gum was insufficient to determine if it is effective at preventing caries.[118][119][120]
Other measures

The use of dental sealants is a means of prevention.[121] A sealant is a thin plastic-like coating applied to the chewing surfaces of the molars to prevent food from being trapped inside pits and fissures. This deprives resident plaque bacteria of carbohydrate, preventing the formation of pit and fissure caries. Sealants are usually applied on the teeth of children, as soon as the teeth erupt but adults are receiving them if not previously performed. Sealants can wear out and fail to prevent access of food and plaque bacteria inside pits and fissures and need to be replaced so they must be checked regularly by dental professionals. Dental sealants have been shown to be more effective at preventing occlusal decay when compared to fluoride varnish applications.[122]
Calcium, as found in food such as milk and green vegetables, is often recommended to protect against dental caries. Fluoride helps prevent decay of a tooth by binding to the hydroxyapatite crystals in enamel.[123] Streptococcus mutans is the leading cause of tooth decay. Low concentration fluoride ions act as bacteriostatic therapeutic agent and high concentration fluoride ions are bactericidal.[124] The incorporated fluorine makes enamel more resistant to demineralization and, thus, resistant to decay.[125] Fluoride can be found in either topical or systemic form.[126] Topical fluoride is more recommended than systemic intake to protect the surface of the teeth.[127] Topical fluoride is used in toothpaste, mouthwash and fluoride varnish.[126] Standard fluoride toothpaste (1,000–1,500 ppm) is more effective than low fluoride toothpaste (< 600ppm) to prevent dental caries.[128] It is recommended that all adult patients to use fluoridated toothpaste with at least 1350ppm fluoride content, brushing at least 2 times per day and brush right before bed. For children and young adults, use fluoridated toothpaste with 1350ppm to 1500ppm fluoride content, brushing 2 times per day and also brush right before bed. American Dental Association Council suggest that for children <3 years old, caregivers should begin brushing their teeth by using fluoridated toothpaste with an amount no more than a smear. Supervised toothbrushing must also be done to children below 8 years of age to prevent swallowing of toothpaste.[129] After brushing with fluoride toothpaste, rinsing should be avoided and the excess spat out.[130] Many dental professionals include application of topical fluoride solutions as part of routine visits and recommend the use of xylitol and amorphous calcium phosphate products. Silver diamine fluoride may work better than fluoride varnish to prevent cavities.[131] Systemic fluoride is found as lozenges, tablets, drops and water fluoridation. These are ingested orally to provide fluoride systemically.[126] Water fluoridation has been shown to be beneficial to prevent tooth decay, especially in low social economical areas, where other forms of fluoride is not available. However, a Cochrane systematic review found no evidence to suggest that taking fluoride systemically daily in pregnant women was effective in preventing dental decay in their offspring.[126]
An oral health assessment carried out before a child reaches the age of one may help with management of caries. The oral health assessment should include checking the child's history, a clinical examination, checking the risk of caries in the child including the state of their occlusion and assessing how well equipped the child's parent or carer is to help the child prevent caries.[132] In order to further increase a child's cooperation in caries management, good communication by the dentist and the rest of the staff of a dental practice should be used. This communication can be improved by calling the child by their name, using eye contact and including them in any conversation about their treatment.[132]
Vaccines are also under development.[133]
Treatment
| No carious lesion | No treatment | ||
| Carious lesion | Inactive lesion | No treatment | |
| Active lesion | Non-cavitated lesion | Non-operative treatment | |
| Cavitated lesion | Operative treatment | ||
| Existing filling | No defect | No replacement | |
| Defective filling | Ditching, overhang | No replacement | |
| Fracture or food impaction | Repair or replacement of filling | ||
| Inactive lesion | No treatment | ||
| Active lesion | Non-cavitated lesion | Non-operative treatment | |
| Cavitated lesion | Repair or replacement of filling |
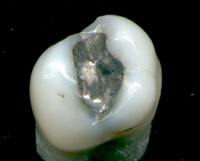
Most importantly, whether the carious lesion is cavitated or non-cavitated dictates the management. Clinical assessment of whether the lesion is active or arrested is also important. Noncavitated lesions can be arrested and remineralization can occur under the right conditions. However, this may require extensive changes to the diet (reduction in frequency of refined sugars), improved oral hygiene (toothbrushing twice per day with fluoride toothpaste and daily flossing), and regular application of topical fluoride. More recently, Immunoglobulin Y specific to Streptococcus mutans has been used to suppress growth of S mutans.[134] Such management of a carious lesion is termed "non-operative" since no drilling is carried out on the tooth. Non-operative treatment requires excellent understanding and motivation from the individual, otherwise the decay will continue.
Once a lesion has cavitated, especially if dentin is involved, remineralization is much more difficult and a dental restoration is usually indicated ("operative treatment"). Before a restoration can be placed, all of the decay must be removed otherwise it will continue to progress underneath the filling. Sometimes a small amount of decay can be left if it is entombed and there is a seal which isolates the bacteria from their substrate. This can be likened to placing a glass container over a candle, which burns itself out once the oxygen is used up. Techniques such as stepwise caries removal are designed to avoid exposure of the dental pulp and overall reduction of the amount of tooth substance which requires removal before the final filling is placed. Often enamel which overlies decayed dentin must also be removed as it is unsupported and susceptible to fracture. The modern decision-making process with regards the activity of the lesion, and whether it is cavitated, is summarized in the table.[135]
Destroyed tooth structure does not fully regenerate, although remineralization of very small carious lesions may occur if dental hygiene is kept at optimal level.[16] For the small lesions, topical fluoride is sometimes used to encourage remineralization. For larger lesions, the progression of dental caries can be stopped by treatment. The goal of treatment is to preserve tooth structures and prevent further destruction of the tooth. Aggressive treatment, by filling, of incipient carious lesions, places where there is superficial damage to the enamel, is controversial as they may heal themselves, while once a filling is performed it will eventually have to be redone and the site serves as a vulnerable site for further decay.[14]
In general, early treatment is quicker and less expensive than treatment of extensive decay. Local anesthetics, nitrous oxide ("laughing gas"), or other prescription medications may be required in some cases to relieve pain during or following treatment or to relieve anxiety during treatment.[136] A dental handpiece ("drill") is used to remove large portions of decayed material from a tooth. A spoon, a dental instrument used to carefully remove decay, is sometimes employed when the decay in dentin reaches near the pulp.[137] Some dentists remove dental caries using a laser rather than the traditional dental drill. A Cochrane review of this technique looked at Er:YAG (erbium-doped yttrium aluminium garnet), Er,Cr:YSGG (erbium, chromium: yttrium-scandium-gallium-garnet) and Nd:YAG (neodymium-doped yttrium aluminium garnet) lasers and found that although people treated with lasers (compared to a conventional dental "drill") experienced less pain and had a lesser need for dental anaesthesia, that overall there was little difference in caries removal.[138] Once the caries is removed, the missing tooth structure requires a dental restoration of some sort to return the tooth to function and aesthetic condition.
Restorative materials include dental amalgam, composite resin, porcelain, and gold.[139] Composite resin and porcelain can be made to match the color of a patient's natural teeth and are thus used more frequently when aesthetics are a concern. Composite restorations are not as strong as dental amalgam and gold; some dentists consider the latter as the only advisable restoration for posterior areas where chewing forces are great.[140] When the decay is too extensive, there may not be enough tooth structure remaining to allow a restorative material to be placed within the tooth. Thus, a crown may be needed. This restoration appears similar to a cap and is fitted over the remainder of the natural crown of the tooth. Crowns are often made of gold, porcelain, or porcelain fused to metal.
For children, preformed crowns are available to place over the tooth. These are usually made of metal (usually stainless steel but increasingly there are aesthetic materials). Traditionally teeth are shaved down to make room for the crown but, more recently, stainless steel crowns have been used to seal decay into the tooth and stop it progressing. This is known as the Hall Technique and works by depriving the bacteria in the decay of nutrients and making their environment less favorable for them. It is a minimally invasive method of managing decay in children and does not require local anesthetic injections in the mouth.

In certain cases, endodontic therapy may be necessary for the restoration of a tooth.[141] Endodontic therapy, also known as a "root canal", is recommended if the pulp in a tooth dies from infection by decay-causing bacteria or from trauma. In root canal therapy, the pulp of the tooth, including the nerve and vascular tissues, is removed along with decayed portions of the tooth. The canals are instrumented with endodontic files to clean and shape them, and they are then usually filled with a rubber-like material called gutta percha.[142] The tooth is filled and a crown can be placed. Upon completion of root canal therapy, the tooth is non-vital, as it is devoid of any living tissue.
An extraction can also serve as treatment for dental caries. The removal of the decayed tooth is performed if the tooth is too far destroyed from the decay process to effectively restore the tooth. Extractions are sometimes considered if the tooth lacks an opposing tooth or will probably cause further problems in the future, as may be the case for wisdom teeth.[143] Extractions may also be preferred by people unable or unwilling to undergo the expense or difficulties in restoring the tooth.
Epidemiology

| no data <50 50–60 60–70 70–80 80–90 90–100 | 100–115 115–130 130–138 138–140 140–142 >142 |
Worldwide, approximately 3.6 billion people have dental caries in their permanent teeth.[7] In baby teeth it affects about 620 million people or 9% of the population.[11] The disease is most common in Latin American countries, countries in the Middle East, and South Asia, and least prevalent in China.[145] In the United States, dental caries is the most common chronic childhood disease, being at least five times more common than asthma.[146] It is the primary pathological cause of tooth loss in children.[147] Between 29% and 59% of adults over the age of 50 experience caries.[148]
Treating dental cavities costs 5–10% of health-care budgets in industrialized countries, and can easily exceed budgets in lower-income countries.[149]
The number of cases has decreased in some developed countries, and this decline is usually attributed to increasingly better oral hygiene practices and preventive measures such as fluoride treatment.[150] Nonetheless, countries that have experienced an overall decrease in cases of tooth decay continue to have a disparity in the distribution of the disease.[148] Among children in the United States and Europe, twenty percent of the population endures sixty to eighty percent of cases of dental caries.[151] A similarly skewed distribution of the disease is found throughout the world with some children having none or very few caries and others having a high number.[148] Australia, Nepal, and Sweden (where children receive dental care paid for by the government) have a low incidence of cases of dental caries among children, whereas cases are more numerous in Costa Rica and Slovakia.[152]
The classic DMF (decay/missing/filled) index is one of the most common methods for assessing caries prevalence as well as dental treatment needs among populations. This index is based on in-field clinical examination of individuals by using a probe, mirror and cotton rolls. Because the DMF index is done without X-ray imaging, it underestimates real caries prevalence and treatment needs.[91]
Bacteria typically associated with dental caries have been isolated from vaginal samples from females who have bacterial vaginosis.[153]
History

There is a long history of dental caries. Over a million years ago, hominins such as Paranthropus suffered from cavities.[154] The largest increases in the prevalence of caries have been associated with dietary changes.[155][156]
Archaeological evidence shows that tooth decay is an ancient disease dating far into prehistory. Skulls dating from a million years ago through the Neolithic period show signs of caries, including those from the Paleolithic and Mesolithic ages.[157] The increase of caries during the neolithic period may be attributed to the increased consumption of plant foods containing carbohydrates.[158] The beginning of rice cultivation in South Asia is also believed to have caused an increase in caries especially for women,[159] although there is also some evidence from sites in Thailand, such as Khok Phanom Di, that shows a decrease in overall percentage of dental caries with the increase in dependence on rice agriculture.[160]
A Sumerian text from 5000 BC describes a "tooth worm" as the cause of caries.[161] Evidence of this belief has also been found in India, Egypt, Japan, and China.[156] Unearthed ancient skulls show evidence of primitive dental work. In Pakistan, teeth dating from around 5500 BC to 7000 BC show nearly perfect holes from primitive dental drills.[162] The Ebers Papyrus, an Egyptian text from 1550 BC, mentions diseases of teeth.[161] During the Sargonid dynasty of Assyria during 668 to 626 BC, writings from the king's physician specify the need to extract a tooth due to spreading inflammation.[156] In the Roman Empire, wider consumption of cooked foods led to a small increase in caries prevalence.[151] The Greco-Roman civilization, in addition to the Egyptian civilization, had treatments for pain resulting from caries.[156]
The rate of caries remained low through the Bronze Age and Iron Age, but sharply increased during the Middle Ages.[155] Periodic increases in caries prevalence had been small in comparison to the 1000 AD increase, when sugar cane became more accessible to the Western world. Treatment consisted mainly of herbal remedies and charms, but sometimes also included bloodletting.[163] The barber surgeons of the time provided services that included tooth extractions.[156] Learning their training from apprenticeships, these health providers were quite successful in ending tooth pain and likely prevented systemic spread of infections in many cases. Among Roman Catholics, prayers to Saint Apollonia, the patroness of dentistry, were meant to heal pain derived from tooth infection.[164]
There is also evidence of caries increase in North American Indians after contact with colonizing Europeans. Before colonization, North American Indians subsisted on hunter-gatherer diets, but afterward there was a greater reliance on maize agriculture, which made these groups more susceptible to caries.[155]
During the European Age of Enlightenment, the belief that a "tooth worm" caused caries was also no longer accepted in the European medical community.[165] Pierre Fauchard, known as the father of modern dentistry, was one of the first to reject the idea that worms caused tooth decay and noted that sugar was detrimental to the teeth and gingiva.[166] In 1850, another sharp increase in the prevalence of caries occurred and is believed to be a result of widespread diet changes.[156] Prior to this time, cervical caries was the most frequent type of caries, but increased availability of sugar cane, refined flour, bread, and sweetened tea corresponded with a greater number of pit and fissure caries.
In the 1890s, W. D. Miller conducted a series of studies that led him to propose an explanation for dental caries that was influential for current theories. He found that bacteria inhabited the mouth and that they produced acids that dissolved tooth structures when in the presence of fermentable carbohydrates.[167] This explanation is known as the chemoparasitic caries theory.[168] Miller's contribution, along with the research on plaque by G. V. Black and J. L. Williams, served as the foundation for the current explanation of the etiology of caries.[156] Several of the specific strains of lactobacilli were identified in 1921 by Fernando E. Rodríguez Vargas.
In 1924 in London, Killian Clarke described a spherical bacterium in chains isolated from carious lesions which he called Streptococcus mutans. Although Clarke proposed that this organism was the cause of caries, the discovery was not followed up. Later, in 1954 in the US, Frank Orland working with hamsters showed that caries was transmissible and caused by acid-producing Streptococcus thus ending the debate whether dental caries were resultant from bacteria. It was not until the late 1960s that it became generally accepted that the Streptococcus isolated from hamster caries was the same as S. mutans.[169]
Tooth decay has been present throughout human history, from early hominids millions of years ago, to modern humans.[170] The prevalence of caries increased dramatically in the 19th century, as the Industrial Revolution made certain items, such as refined sugar and flour, readily available.[156] The diet of the “newly industrialized English working class”[156] then became centered on bread, jam, and sweetened tea, greatly increasing both sugar consumption and caries.
Etymology and usage
Naturalized from Latin into English (a loanword), caries in its English form originated as a mass noun that means "rottenness",[3][171] that is, "decay".
Cariesology[172][173][174] or cariology[175] is the study of dental caries.
Society and culture
It is estimated that untreated dental caries results in worldwide productivity losses in the size of about US$27 billion yearly.[176]
Other animals
Dental caries is uncommon among companion animals.[177]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Laudenbach, JM; Simon, Z (November 2014). "Common Dental and Periodontal Diseases: Evaluation and Management". The Medical Clinics of North America. 98 (6): 1239–1260. doi:10.1016/j.mcna.2014.08.002. PMID 25443675.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Oral health Fact sheet N°318". who.int. April 2012. Archived from the original on 8 December 2014. Retrieved 10 December 2014.
- ↑ 3.0 3.1 3.2 3.3 Taber's cyclopedic medical dictionary (Ed. 22, illustrated in full color ed.). Philadelphia: F.A. Davis Co. 2013. p. 401. ISBN 9780803639096. Archived from the original on 2015-07-13.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 SECTION ON ORAL, HEALTH; SECTION ON ORAL, HEALTH (December 2014). "Maintaining and improving the oral health of young children". Pediatrics. 134 (6): 1224–9. doi:10.1542/peds.2014-2984. PMID 25422016.
- ↑ 5.0 5.1 de Oliveira, KMH; Nemezio, MA; Romualdo, PC; da Silva, RAB; de Paula E Silva, FWG; Küchler, EC (2017). "Dental Flossing and Proximal Caries in the Primary Dentition: A Systematic Review". Oral Health & Preventive Dentistry. 15 (5): 427–434. doi:10.3290/j.ohpd.a38780. PMID 28785751.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 Silk, H (March 2014). "Diseases of the mouth". Primary Care: Clinics in Office Practice. 41 (1): 75–90. doi:10.1016/j.pop.2013.10.011. PMID 24439882.
- ↑ 7.0 7.1 7.2 "Oral health". www.who.int. Archived from the original on 2019-10-17. Retrieved 2019-09-14.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Schwendicke, F; Dörfer, CE; Schlattmann, P; Page, LF; Thomson, WM; Paris, S (January 2015). "Socioeconomic Inequality and Caries: A Systematic Review and Meta-Analysis". Journal of Dental Research. 94 (1): 10–18. doi:10.1177/0022034514557546. PMID 25394849.
- ↑ US Preventive Services Task, Force.; Davidson, KW; Barry, MJ; Mangione, CM; Cabana, M; Caughey, AB; Davis, EM; Donahue, KE; Doubeni, CA; Kubik, M; Li, L; Ogedegbe, G; Pbert, L; Silverstein, M; Stevermer, J; Tseng, CW; Wong, JB (7 December 2021). "Screening and Interventions to Prevent Dental Caries in Children Younger Than 5 Years: US Preventive Services Task Force Recommendation Statement". JAMA. 326 (21): 2172–2178. doi:10.1001/jama.2021.20007. PMID 34874412.
- ↑ Otsu, K; Kumakami-Sakano, M; Fujiwara, N; Kikuchi, K; Keller, L; Lesot, H; Harada, H (2014). "Stem cell sources for tooth regeneration: current status and future prospects". Frontiers in Physiology. 5: 36. doi:10.3389/fphys.2014.00036. PMC 3912331. PMID 24550845.
- ↑ 11.0 11.1 Vos, T (Dec 15, 2012). "Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010". The Lancet. 380 (9859): 2163–96. doi:10.1016/S0140-6736(12)61729-2. PMC 6350784. PMID 23245607.
- ↑ Bagramian, RA; Garcia-Godoy, F; Volpe, AR (February 2009). "The global increase in dental caries. A pending public health crisis". American Journal of Dentistry. 22 (1): 3–8. PMID 19281105.
- ↑ Health Promotion Board: Dental Caries Archived 2010-09-01 at the Wayback Machine, affiliated with the Singapore government. Page accessed August 14, 2006.
- ↑ 14.0 14.1 Richie S. King (November 28, 2011). "A Closer Look at Teeth May Mean More Fillings". The New York Times. Archived from the original on November 29, 2011. Retrieved November 30, 2011.
An incipient carious lesion is the initial stage of structural damage to the enamel, usually caused by a bacterial infection that produces tooth-dissolving acid.
- ↑ Johnson, Clarke. "Biology of the Human Dentition Archived 2015-10-30 at the Wayback Machine." Page accessed July 18, 2007.
- ↑ 16.0 16.1 MedlinePlus Encyclopedia: Dental Cavities
- ↑ Tooth Decay, hosted on the New York University Medical Center website. Page accessed August 14, 2006.
- ↑ Cavernous Sinus Thrombosis Archived 2008-05-27 at the Wayback Machine, hosted on WebMD. Page accessed May 25, 2008.
- ↑ MedlinePlus Encyclopedia: Ludwig's Anigna
- ↑ Hartmann, Richard W. Ludwig's Angina in Children Archived 2008-07-09 at the Wayback Machine, hosted on the American Academy of Family Physicians website. Page accessed May 25, 2008.
- ↑ Southam JC, Soames JV (1993). "2. Dental Caries". Oral pathology (2nd ed.). Oxford: Oxford Univ. Press. ISBN 978-0-19-262214-3.
- ↑ Wong, Allen; Young, Douglas A.; Emmanouil, Dimitris E.; Wong, Lynne M.; Waters, Ashley R.; Booth, Mark T. (2013-06-01). "Raisins and oral health". Journal of Food Science. 78 Suppl 1: A26–29. doi:10.1111/1750-3841.12152. ISSN 1750-3841. PMID 23789933.
- ↑ Smith B, Pickard HM, Kidd EA (1990). "1. Why restore teeth?". Pickard's manual of operative dentistry (6th ed.). Oxford University Press. ISBN 978-0-19-261808-5.
- ↑ 24.0 24.1 Hardie JM (May 1982). "The microbiology of dental caries". Dental Update. 9 (4): 199–200, 202–4, 206–8. PMID 6959931.
- ↑ 25.0 25.1 Holloway PJ; Moore, W.J. (September 1983). "The role of sugar in the etiology of dental caries". Journal of Dentistry. 11 (3): 189–213. doi:10.1016/0300-5712(83)90182-3. PMID 6358295.
- ↑ Watt RG, Listl S, Peres MA, Heilmann A, editors. Social inequalities in oral health: from evidence to action Archived 2015-06-19 at the Wayback Machine. London: International Centre for Oral Health Inequalities Research & Policy; www.icohirp.com
- ↑ Marsh, Philip D.; Head, David A.; Devine, Deirdre A. (2015). "Dental plaque as a biofilm and a microbial community—Implications for treatment". Journal of Oral Biosciences. 57 (4): 185–191. doi:10.1016/j.job.2015.08.002. Archived from the original on 29 August 2021. Retrieved 4 August 2020.
{{cite journal}}: More than one of|accessdate=and|access-date=specified (help); More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Marsh, P (1994). "Microbial ecology of dental plaque and its significance in health and disease". Advances in Dental Research. 8 (2): 263–71. doi:10.1177/08959374940080022001. PMID 7865085.
- ↑ Cavities/tooth decay Archived 2008-03-15 at the Wayback Machine, hosted on the Mayo Clinic website. Page accessed May 25, 2008.
- ↑ Douglass, JM; Li, Y; Tinanoff, N. (Sep–Oct 2008), "Association of mutans streptococci between caregivers and their children", Pediatric Dentistry, 30 (5): 375–87, PMID 18942596
- ↑ Silverstone LM (May 1983). "Remineralization and enamel caries: new concepts". Dental Update. 10 (4): 261–73. PMID 6578983.
- ↑ Madigan M.T. & Martinko J.M. Brock – Biology of Microorganisms. 11th Ed., 2006, Pearson, USA. pp. 705
- ↑ Dental Caries Archived 2006-06-30 at the Wayback Machine, hosted on the University of California Los Angeles School of Dentistry website. Page accessed August 14, 2006.
- ↑ Summit, James B., J. William Robbins, and Richard S. Schwartz. "Fundamentals of Operative Dentistry: A Contemporary Approach." 2nd edition. Carol Stream, Illinois, Quintessence Publishing Co, Inc, 2001, p. 75. ISBN 0-86715-382-2.
- ↑ 35.0 35.1 Mast P, Rodrigueztapia MT, Daeniker L, Krejci I (Sep 2013). "Understanding MIH: definition, epidemiology, differential diagnosis and new treatment guidelines" (PDF). European Journal of Paediatric Dentistry (Review). 14 (3): 204–8. PMID 24295005. Archived (PDF) from the original on 2016-10-05.
- ↑ Silva, Mihiri J.; Scurrah, Katrina J.; Craig, Jeffrey M.; Manton, David J.; Kilpatrick, Nicky (August 2016). "Etiology of molar incisor hypomineralization - A systematic review". Community Dentistry and Oral Epidemiology. 44 (4): 342–353. doi:10.1111/cdoe.12229. ISSN 1600-0528. PMID 27121068.
- ↑ William V, Messer LB, Burrow MF (2006). "Molar incisor hypomineralization: review and recommendations for clinical management" (PDF). Pediatric Dentistry (Review). 28 (3): 224–32. PMID 16805354. Archived (PDF) from the original on 2016-03-06.
- ↑ "Dental Enamel Defects and Celiac Disease" (PDF). National Institute of Health (NIH). Archived (PDF) from the original on 2016-03-05. Retrieved Mar 7, 2016.
Tooth defects that result from celiac disease may resemble those caused by too much fluoride or a maternal or early childhood illness. Dentists mostly say it’s from fluoride, that the mother took tetracycline, or that there was an illness early on
- ↑ Ferraz EG, Campos Ede J, Sarmento VA, Silva LR (2012). "The oral manifestations of celiac disease: information for the pediatric dentist". Pediatric Dentistry (Review). 34 (7): 485–8. PMID 23265166.
The presence of these clinical features in children may signal the need for early investigation of possible celiac disease, especially in asymptomatic cases. (...) Pediatric dentists must recognize typical oral lesions, especially those associated with nutritional deficiencies, and should suspect the presence of celiac disease, which can change the disease’s course and patient’s prognosis.
- ↑ Rashid M, Zarkadas M, Anca A, Limeback H (2011). "Oral manifestations of celiac disease: a clinical guide for dentists". Journal of the Canadian Dental Association (Review). 77: b39. PMID 21507289. Archived from the original on 2016-03-08.
- ↑ Giuca MR, Cei G, Gigli F, Gandini P (2010). "Oral signs in the diagnosis of celiac disease: review of the literature". Minerva Stomatologica (Review). 59 (1–2): 33–43. PMID 20212408.
- ↑ Neville, B.W., Damm, Douglas; Allen, Carl and Bouquot, Jerry (2002). "Oral & Maxillofacial Pathology." 2nd edition, p. 89. ISBN 0-7216-9003-3.
- ↑ Neville, B.W., Damm, Douglas; Allen, Carl and Bouquot, Jerry (2002). "Oral & Maxillofacial Pathology." 2nd edition, p. 94. ISBN 0-7216-9003-3.
- ↑ Nanci, p. 122
- ↑ Dawes C (December 2003). "What is the critical pH and why does a tooth dissolve in acid?". Journal of the Canadian Dental Association. 69 (11): 722–4. PMID 14653937. Archived from the original on 2009-07-14.
- ↑ Mellberg JR (1986). "Demineralization and remineralization of root surface caries". Gerodontology. 5 (1): 25–31. doi:10.1111/j.1741-2358.1986.tb00380.x. PMID 3549537.
- ↑ Borzabadi-Farahani, A; Eslamipour, F; Asgari, I (2011). "Association between orthodontic treatment need and caries experience". Acta Odontologica Scandinavica. 69 (1): 2–11. doi:10.3109/00016357.2010.516732. PMID 20923258.
- ↑ Hafez, HS; Shaarawy, SM; Al-Sakiti, AA; Mostafa, YA (2012). "Dental crowding as a caries risk factor: A systematic review". American Journal of Orthodontics and Dentofacial Orthopedics. 142 (4): 443–50. doi:10.1016/j.ajodo.2012.04.018. PMID 22999666.
- ↑ 49.0 49.1 Neville, B. W., Douglas Damm, Carl Allen, Jerry Bouquot. Oral & Maxillofacial Pathology 2nd edition, 2002, p. 398. ISBN 0-7216-9003-3.
- ↑ Oral Complications of Chemotherapy and Head/Neck Radiation Archived 2008-12-06 at the Wayback Machine, hosted on the National Cancer Institute Archived 2015-03-12 at the Wayback Machine website. Page accessed January 8, 2007.
- ↑ See Common effects of cancer therapies on salivary glands at "Archived copy". Archived from the original on 2013-12-02. Retrieved 2013-07-30.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Ralph R. Steinman & John Leonora (1971) "Relationship of fluid transport through dentation to the incidence of dental caries", Journal of Dental Research 50(6): 1536 to 43
- ↑ Neville, B.W., Douglas Damm, Carl Allen, Jerry Bouquot. Oral & Maxillofacial Pathology 2nd edition, 2002, p. 347. ISBN 0-7216-9003-3.
- ↑ Tobacco Use Increases the Risk of Gum Disease Archived 2007-01-09 at the Wayback Machine, hosted on the American Academy of Periodontology Archived 2005-12-14 at the Wayback Machine. Page accessed January 9, 2007.
- ↑ Banting, D. W. "The Diagnosis of Root Caries Archived 2006-09-30 at the Wayback Machine." Presentation to the National Institute of Health Consensus Development Conference on Diagnosis and Management of Dental Caries Throughout Life, in pdf format, hosted on the National Institute of Dental and Craniofacial Research, p. 19. Page accessed August 15, 2006.
- ↑ Executive Summary Archived 2007-02-16 at the Wayback Machine of U.S. Surgeon General's report titled, "The Health Consequences of Smoking: A Report of the Surgeon General," hosted on the CDC Archived 2012-03-20 at the Wayback Machine website, p. 12. Page accessed January 9, 2007.
- ↑ Zhou, S; Rosenthal, DG; Sherman, S; Zelikoff, J; Gordon, T; Weitzman, M (September 2014). "Physical, behavioral, and cognitive effects of prenatal tobacco and postnatal secondhand smoke exposure". Current Problems in Pediatric and Adolescent Health Care. 44 (8): 219–41. doi:10.1016/j.cppeds.2014.03.007. PMC 6876620. PMID 25106748.
- ↑ Brudevold F, Steadman LT (1956). "The distribution of lead in human enamel". Journal of Dental Research. 35 (3): 430–437. doi:10.1177/00220345560350031401. PMID 13332147.
- ↑ Brudevold F, Aasenden R, Srinivasian BN, Bakhos Y (1977). "Lead in enamel and saliva, dental caries and the use of enamel biopsies for measuring past exposure to lead". Journal of Dental Research. 56 (10): 1165–1171. doi:10.1177/00220345770560100701. PMID 272374.
- ↑ Goyer RA (1990). "Transplacental transport of lead". Environmental Health Perspectives. 89: 101–105. doi:10.2307/3430905. JSTOR 3430905. PMC 1567784. PMID 2088735.
- ↑ Moss ME, Lanphear BP, Auinger P (1999). "Association of dental caries and blood lead levels". JAMA. 281 (24): 2294–8. doi:10.1001/jama.281.24.2294. PMID 10386553.
- ↑ Campbell JR, Moss ME, Raubertas RF (2000). "The association between caries and childhood lead exposure". Environmental Health Perspectives. 108 (11): 1099–1102. doi:10.2307/3434965. JSTOR 3434965. PMC 1240169. PMID 11102303.
- ↑ Gemmel A, Tavares M, Alperin S, Soncini J, Daniel D, Dunn J, Crawford S, Braveman N, Clarkson TW, McKinlay S, Bellinger DC (2002). "Blood Lead Level and Dental Caries in School-Age Children". Environmental Health Perspectives. 110 (10): A625–A630. doi:10.1289/ehp.021100625. PMC 1241049. PMID 12361944.
- ↑ Billings RJ, Berkowitz RJ, Watson G (2004). "Teeth". Pediatrics. 113 (4): 1120–1127. PMID 15060208.
- ↑ Leroy N, Bres E (2001). "Structure and substitutions in fluorapatite". European Cells and Materials. 2: 36–48. doi:10.22203/eCM.v002a05. PMID 14562256.
- ↑ Arora M, Weuve J, Schwartz J, Wright RO (2008). "Association of environmental cadmium exposure with pediatric dental caries". Environmental Health Perspectives. 116 (6): 821–825. doi:10.1289/ehp.10947. PMC 2430240. PMID 18560540.
- ↑ Dye B (2010). "Trends in Oral Health by Poverty Status as Measured by Healthy People 2010 Objectives". Public Health Reports. 125 (6): 817–30. doi:10.1177/003335491012500609. PMC 2966663. PMID 21121227.
- ↑ Selwitz R. H.; Ismail A. I.; Pitts N. B. (2007). "Dental caries". The Lancet. 369 (9555): 51–59. doi:10.1016/s0140-6736(07)60031-2. PMID 17208642.
- ↑ ADA Caries Risk Assessment Form Completion Instructions. American Dental Association
- ↑ Tellez, M., Gomez, J., Pretty, I., Ellwood, R., Ismail, A. (2013). "Evidence on existing caries risk assessment systems: are they predictive of future caries?". Community Dentistry and Oral Epidemiology. 41 (1): 67–78. doi:10.1111/cdoe.12003. PMID 22978796.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Herbst RS (2004). "Review of epidermal growth factor receptor biology". International Journal of Radiation Oncology*biology*physics. 59 (2 Suppl): 21–6. doi:10.1016/j.ijrobp.2003.11.041. PMID 15142631.
- ↑ Venturi S, Venturi M (2009). "Iodine in evolution of salivary glands and in oral health". Nutrition and Health. 20 (2): 119–134. doi:10.1177/026010600902000204. PMID 19835108.
- ↑ Dental caries : the disease and its clinical management. Fejerskov, Ole., Kidd, Edwina A. M. (2nd ed.). Oxford: Blackwell Munksgaard. 2008. pp. 166–169. ISBN 978-1-4051-3889-5. OCLC 136316302.
{{cite book}}: CS1 maint: others (link) - ↑ Banerjee, Avijit. (2011). Pickard's manual of operative dentistry. Watson, Timothy F. (Ninth ed.). Oxford. p. 2. ISBN 978-0-19-100304-2. OCLC 867050322.
- ↑ Fejerskov O, Nyvad B, Kidd EA (2008) "Pathology of dental caries", pp 20–48 in Fejerskov O, Kidd EAM (eds) Dental caries: The disease and its clinical management. Oxford, Blackwell Munksgaard, Vol. 2. ISBN 1444309285.
- ↑ 76.0 76.1 Kidd EA, Fejerskov O (2004). "What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms". Journal of Dental Research. 83 Spec No C: C35–8. doi:10.1177/154405910408301S07. PMID 15286119.
- ↑ Darling AI (1963). "Resistance of the enamel to dental caries". Journal of Dental Research. 42 (1 Pt2): 488–96. doi:10.1177/00220345630420015601. PMID 14041429.
- ↑ Robinson C, Shore RC, Brookes SJ, Strafford S, Wood SR, Kirkham J (2000). "The chemistry of enamel caries". Critical Reviews in Oral Biology & Medicine. 11 (4): 481–95. doi:10.1177/10454411000110040601. PMID 11132767.
- ↑ Nanci, p. 121
- ↑ "Teeth & Jaws: Caries, Pulp, & Periapical Conditions Archived 2007-05-06 at the Wayback Machine," hosted on the University of Southern California School of Dentistry Archived 2005-12-07 at the Wayback Machine website. Page accessed June 22, 2007.
- ↑ Ross, Michael H., Kaye, Gordon I. and Pawlina, Wojciech (2003) Histology: a text and atlas. 4th edition, p. 450. ISBN 0-683-30242-6.
- ↑ 82.0 82.1 Nanci, p. 166
- ↑ Summit, James B., J. William Robbins, and Richard S. Schwartz. Fundamentals of Operative Dentistry: A Contemporary Approach 2nd edition. Carol Stream, Illinois, Quintessence Publishing Co, Inc, 2001, p. 13. ISBN 0-86715-382-2.
- ↑ Dababneh RH, Khouri AT, Addy M (December 1999). "Dentine hypersensitivity – an enigma? A review of terminology, mechanisms, aetiology and management". British Dental Journal. 187 (11): 606–11, discussion 603. doi:10.1038/sj.bdj.4800345a. PMID 16163281.
- ↑ 85.0 85.1 Smith AJ, Murray PE, Sloan AJ, Matthews JB, Zhao S (August 2001). "Trans-dentinal stimulation of tertiary dentinogenesis". Advances in Dental Research. 15: 51–4. doi:10.1177/08959374010150011301. PMID 12640740.
- ↑ Summit, James B., J. William Robbins, and Richard S. Schwartz. "Fundamentals of Operative Dentistry: A Contemporary Approach." 2nd edition. Carol Stream, Illinois, Quintessence Publishing Co, Inc, 2001, p. 14. ISBN 0-86715-382-2.
- ↑ Illustrated Embryology, Histology, and Anatomy, Bath-Balogh and Fehrenbach, Elsevier, 2011[page needed]
- ↑ 88.0 88.1 Deana, A M; Jesus, S H C; Koshoji, N H; Bussadori, S K; Oliveira, M T (2013). "Detection of early carious lesions using contrast enhancement with coherent light scattering (speckle imaging)". Laser Physics. 23 (7): 075607. Bibcode:2013LaPhy..23g5607D. doi:10.1088/1054-660x/23/7/075607.
- ↑ Rosenstiel, Stephen F. Clinical Diagnosis of Dental Caries: A North American Perspective Archived 2006-08-09 at the Wayback Machine. Maintained by the University of Michigan Dentistry Library, along with the National Institutes of Health, National Institute of Dental and Craniofacial Research. 2000. Page accessed August 13, 2006.
- ↑ Summit, James B., J. William Robbins, and Richard S. Schwartz. Fundamentals of Operative Dentistry: A Contemporary Approach 2nd edition. Carol Stream, Illinois, Quintessence Publishing Co, Inc, 2001, p. 31. ISBN 0-86715-382-2.
- ↑ 91.0 91.1 Zadik Yehuda; Bechor Ron (June–July 2008). "Hidden Occlusal Caries – Challenge for the Dentist" (PDF). The New York State Dental Journal. 74 (4): 46–50. PMID 18788181. Archived from the original (PDF) on 2011-07-22. Retrieved 2008-08-08.
- ↑ Baelum, edited by Ole Fejerskov and Edwina Kidd; with Bente Nyvad and Vibeke (2008). Dental caries: the disease and its clinical management (2nd ed.). Oxford: Blackwell Munksgaard. p. 67. ISBN 978-1-4051-3889-5.
- ↑ Sonis, Stephen T. (2003). Dental Secrets (3rd ed.). Philadelphia: Hanley & Belfus. p. 130. ISBN 978-1-56053-573-7.
- ↑ Sukumaran Anil. Early Childhood Caries: Prevalence, Risk Factors, and Prevention
- ↑ ADA Early Childhood Tooth Decay (Baby Bottle Tooth Decay) Archived 2006-08-13 at the Wayback Machine. Hosted on the American Dental Association website. Page accessed August 14, 2006.
- ↑ Statement on Early Childhood Caries, American Dental Association at "Statement on Early Childhood Caries". Archived from the original on 2013-05-12. Retrieved 2013-07-30.
- ↑ Radiographic Classification of Caries Archived 2006-08-23 at the Wayback Machine. Hosted on the Ohio State University website. Page accessed August 14, 2006.
- ↑ ADA Methamphetamine Use (METH MOUTH) Archived 2008-06-01 at the Wayback Machine. Hosted on the American Dental Association website. Page accessed February 14, 2007.
- ↑ Prevention and Management of Dental Caries in Children. Dundee Dental Education Centre, Frankland Building, Small's Wynd, Dundee DD1 4HN, Scotland: Scottish Dental Clinical Effectiveness Programme. April 2010. p. 11. ISBN 9781905829088.
{{cite book}}: CS1 maint: location (link) - ↑ Helfenstein, U.; Steiner, M.; Marthaler, T. M. (1991). "Caries prediction on the basis of past caries including precavity lesions". Caries Research. 25 (5): 372–6. doi:10.1159/000261394. PMID 1747888.
- ↑ Seppa, Liisa; Hausen, Hannu; Pollanen, Lea; Helasharju, Kirsti; Karkkainen, Sakari (1989). "Past caries recordings made in Public Dental Clinics as predictors of caries prevalence in early adolescence". Community Dentistry and Oral Epidemiology. 17 (6): 277–281. doi:10.1111/j.1600-0528.1989.tb00635.x. PMID 2686924.
- ↑ Dorri, Mojtaba; Dunne, Stephen M; Walsh, Tanya; Schwendicke, Falk (2015-11-05). "Micro-invasive interventions for managing proximal dental decay in primary and permanent teeth". Cochrane Database of Systematic Reviews (11): CD010431. doi:10.1002/14651858.cd010431.pub2. ISSN 1465-1858. PMID 26545080.
- ↑ Introduction to Dental Plaque Archived 2006-06-23 at the Wayback Machine. Hosted on the Leeds Dental Institute Website. Page accessed August 14, 2006.
- ↑ Hujoel, Philippe Pierre; Hujoel, Margaux Louise A.; Kotsakis, Georgios A. (2018). "Personal oral hygiene and dental caries: A systematic review of randomised controlled trials". Gerodontology. 35 (4): 282–289. doi:10.1111/ger.12331. ISSN 1741-2358. PMID 29766564.
- ↑ Sambunjak, Dario; Nickerson, Jason W; Poklepovic, Tina; Johnson, Trevor M; Imai, Pauline; Tugwell, Peter; Worthington, Helen V (2011-12-07). "Flossing for the management of periodontal diseases and dental caries in adults". Cochrane Database of Systematic Reviews (12): CD008829. doi:10.1002/14651858.cd008829.pub2. ISSN 1465-1858. PMID 22161438.
- ↑ Deacon, Scott A.; Glenny, Anne-Marie; Deery, Chris; Robinson, Peter G.; Heanue, Mike; Walmsley, A Damien; Shaw, William C. (2010). "Different powered toothbrushes for plaque control and gingival health". Cochrane Database of Systematic Reviews (12): CD004971. doi:10.1002/14651858.cd004971.pub2. PMID 21154357.
- ↑ Walsh, Tanya; Worthington, Helen V.; Glenny, Anne-Marie; Marinho, Valeria Cc; Jeroncic, Ana (4 March 2019). "Fluoride toothpastes of different concentrations for preventing dental caries". The Cochrane Database of Systematic Reviews. 3: CD007868. doi:10.1002/14651858.CD007868.pub3. ISSN 1469-493X. PMC 6398117. PMID 30829399.
- ↑ Kraivaphan, Petcharat; Amornchat, Cholticha; Triratana, T; Mateo, L.R.; Ellwood, R; Cummins, Diane; Devizio, William; Zhang, Y-P (2013-08-28). "Two-Year Caries Clinical Study of the Efficacy of Novel Dentifrices Containing 1.5% Arginine, an Insoluble Calcium Compound and 1,450 ppm Fluoride". Caries Research. 47 (6): 582–590. doi:10.1159/000353183. PMID 23988908. Archived from the original on 2020-05-10. Retrieved 2020-08-04.
{{cite journal}}: More than one of|accessdate=and|access-date=specified (help); More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Kraivaphan, P.; Amornchat, C.; Triratana, T.; Mateo, L.R.; Ellwood, R.; Cummins, D.; Devizio, W.; Zhang, Y.-P. (2013). "Two-Year Caries Clinical Study of the Efficacy of Novel Dentifrices Containing 1.5% Arginine, an Insoluble Calcium Compound and 1,450 ppm Fluoride". Caries Research. 47 (6): 582–590. doi:10.1159/000353183. PMID 23988908. Archived from the original on 2018-11-16. Retrieved 2020-08-04.
{{cite journal}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Marinho, Valeria C. C.; Worthington, Helen V.; Walsh, Tanya; Chong, Lee Yee (2015-06-15). "Fluoride gels for preventing dental caries in children and adolescents". Cochrane Database of Systematic Reviews (6): CD002280. doi:10.1002/14651858.CD002280.pub2. ISSN 1469-493X. PMC 7138249. PMID 26075879.
- ↑ Marinho, Valeria C. C.; Chong, Lee Yee; Worthington, Helen V.; Walsh, Tanya (2016-07-29). "Fluoride mouthrinses for preventing dental caries in children and adolescents". Cochrane Database of Systematic Reviews. 7: CD002284. doi:10.1002/14651858.CD002284.pub2. ISSN 1469-493X. PMC 6457869. PMID 27472005.
- ↑ al-Khateeb TL, O'Mullane DM, Whelton H, Sulaiman MI (2003). "Periodontal treatment needs among Saudi Arabian adults and their relationship to the use of the Miswak". Community Dental Health. 8 (4): 323–328. ISSN 0265-539X. PMID 1790476.
- ↑ Sheiham, A; James, WP (October 2014). "A new understanding of the relationship between sugars, dental caries and fluoride use: implications for limits on sugars consumption". Public Health Nutrition. 17 (10): 2176–84. doi:10.1017/S136898001400113X. PMID 24892213.
- ↑ Kashket, S.; Van Houte, J.; Lopez, L. R.; Stocks, S. (1991-10-01). "Lack of correlation between food retention on the human dentition and consumer perception of food stickiness". Journal of Dental Research. 70 (10): 1314–1319. doi:10.1177/00220345910700100101. ISSN 0022-0345. PMID 1939824.
- ↑ "Nutrition and tooth decay in infancy". European Academy of Paediatric Dentistry. Dr. Kyriaki Tsinidou. Archived from the original on 2019-04-06. Retrieved 2019-04-06.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Oral Health Topics: Baby Bottle Tooth Decay Archived 2006-08-13 at the Wayback Machine, hosted on the American Dental Association website. Page accessed August 14, 2006.
- ↑ Guideline on Infant Oral Health Care Archived 2006-12-06 at the Wayback Machine, hosted on the American Academy of Pediatric Dentistry Archived 2007-01-12 at the Wayback Machine website. Page accessed January 13, 2007.
- ↑ Twetman, S (2015). "The evidence base for professional and self-care prevention--caries, erosion and sensitivity". BMC Oral Health. 15 Suppl 1: S4. doi:10.1186/1472-6831-15-S1-S4. PMC 4580782. PMID 26392204.
- ↑ Twetman, S; Dhar, V (2015). "Evidence of Effectiveness of Current Therapies to Prevent and Treat Early Childhood Caries". Pediatric Dentistry. 37 (3): 246–53. PMID 26063553. Archived from the original on 2017-03-28.
- ↑ Riley P, Moore D, Ahmed F, Sharif MO, Worthington HV (March 2015). "Xylitol-containing products for preventing dental caries in children and adults". Cochrane Database of Systematic Reviews (3): CD010743. doi:10.1002/14651858.CD010743.pub2. PMID 25809586.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Mejare I, Lingstrom P, Petersson LG, Holm AK, Twetman S, Kallestal C, Nordenram G, Lagerlof F, Soder B, Norlund A, Axelsson S, Dahlgren H (2003). "Caries-preventive effect of fissure sealants: a systematic review". Acta Odontologica Scandinavica. 61 (6): 321–330. doi:10.1080/00016350310007581. PMID 14960003.
- ↑ Ahovuo-Saloranta, Anneli; Forss, Helena; Hiiri, Anne; Nordblad, Anne; Mäkelä, Marjukka (2016-01-18). "Pit and fissure sealants versus fluoride varnishes for preventing dental decay in the permanent teeth of children and adolescents". The Cochrane Database of Systematic Reviews (1): CD003067. doi:10.1002/14651858.CD003067.pub4. ISSN 1469-493X. PMC 7177291. PMID 26780162.
- ↑ Nanci, p. 7
- ↑ A, Deepti; Jeevarathan, J; Muthu, MS; Prabhu V, Rathna; Chamundeswari (2008-01-01). "Effect of Fluoride Varnish on Streptococcus mutans Count in Saliva of Caries Free Children Using Dentocult SM Strip Mutans Test: A Randomized Controlled Triple Blind Study". International Journal of Clinical Pediatric Dentistry. 1 (1): 1–9. doi:10.5005/jp-journals-10005-1001. ISSN 0974-7052. PMC 4086538. PMID 25206081.
- ↑ Ross, Michael H., Kaye, Gordon I. and Pawlina, Wojciech (2003). Histology: A Text and Atlas. 4th edition, p. 453. ISBN 0-683-30242-6.
- ↑ 126.0 126.1 126.2 126.3 Takahashi, Rena; Ota, Erika; Hoshi, Keika; Naito, Toru; Toyoshima, Yoshihiro; Yuasa, Hidemichi; Mori, Rintaro (2015). Mori, Rintaro (ed.). "Fluoride supplementation in pregnant women for preventing dental caries in the primary teeth of their children". Cochrane Database of Systematic Reviews (8): CD011850. doi:10.1002/14651858.cd011850.
- ↑ Limited evidence suggests fluoride varnish applied twice yearly is effective for caries prevention in children at "Archived copy". Archived from the original on 2013-12-03. Retrieved 2013-07-30.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Santos, A. P. P.; Oliveira, B. H.; Nadanovsky, P. (2013-01-01). "Effects of low and standard fluoride toothpastes on caries and fluorosis: systematic review and meta-analysis". Caries Research. 47 (5): 382–390. doi:10.1159/000348492. ISSN 1421-976X. PMID 23572031.
- ↑ American Dental Association Council on Scientific Affairs (February 2014). "Fluoride toothpaste use for young children". The Journal of the American Dental Association. 145 (2): 190–191. doi:10.14219/jada.2013.47. ISSN 0002-8177. PMID 24487611.
- ↑ "Delivering Better Oral Health: An evidence-based toolkit for prevention, second edition" (PDF). Department of Health / British Association for the Study of Community Dentistry. April 2009. Archived (PDF) from the original on 2010-08-10. Retrieved 2020-08-04.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ "Prevention and management of dental caries in children: Dental clinical guidance" (PDF). sdcep.co.uk. April 2010. pp. 6–7. Archived (PDF) from the original on 5 October 2016. Retrieved 7 March 2016.
- ↑ 132.0 132.1 "Prevention and management of dental caries in children: Dental clinical guidance" (PDF). sdcep.co.uk. April 2010. pp. 6–7. Archived (PDF) from the original on 5 October 2016. Retrieved 7 March 2016.
- ↑ Russell, MW; Childers, NK; Michalek, SM; Smith, DJ; Taubman, MA (May–Jun 2004). "A Caries Vaccine? The state of the science of immunization against dental caries". Caries Research. 38 (3): 230–5. doi:10.1159/000077759. PMID 15153693.
- ↑ Chiba, Itsuo; Isogai, Hiroshi; Kobayashi-Sakamoto, Michiyo; Mizugai, Hiroyuki; Hirose, Kimiharu; Isogai, Emiko; Nakano, Taku; Icatlo, Faustino C.; Nguyen, Sa V. (2011-08-01). "Anti–cell-associated glucosyltransferase immunoglobulin Y suppression of salivary mutans streptococci in healthy young adults". The Journal of the American Dental Association. 142 (8): 943–949. doi:10.14219/jada.archive.2011.0301. ISSN 0002-8177. PMID 21804061. Archived from the original on 2020-02-05. Retrieved 2020-08-04.
{{cite journal}}: More than one of|accessdate=and|access-date=specified (help); More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Ole Fejerskov; Edwina Kidd (2004). Dental caries : the disease and its clinical management. Copenhagen [u.a.]: Blackwell Munksgaard. ISBN 9781405107181.
- ↑ Oral Health Topics: Anesthesia Frequently Asked Questions Archived 2006-07-16 at the Wayback Machine, hosted on the American Dental Association website. Page accessed August 16, 2006.
- ↑ Summit, James B., J. William Robbins, and Richard S. Schwartz. "Fundamentals of Operative Dentistry: A Contemporary Approach." 2nd edition. Carol Stream, Illinois, Quintessence Publishing Co, Inc, 2001, p. 128. ISBN 0-86715-382-2.
- ↑ Montedori, Alessandro; Abraha, Iosief; Orso, Massimiliano; d'Errico, Potito Giuseppe; Pagano, Stefano; Lombardo, Guido (2016). "Lasers for caries removal in deciduous and permanent teeth". Cochrane Database of Systematic Reviews. 9: CD010229. doi:10.1002/14651858.cd010229.pub2. PMC 6457657. PMID 27666123.
- ↑ "Aspects of Treatment of Cavities and of Caries Disease Archived 2008-12-07 at the Wayback Machine" from the Disease Control Priorities Project. Page accessed August 15, 2006.
- ↑ Oral Health Topics: Dental Filling Options Archived 2009-08-30 at the Wayback Machine, hosted on the American Dental Association website. Page accessed August 16, 2006.
- ↑ What is a Root Canal? Archived 2006-08-06 at the Wayback Machine, hosted by the Academy of General Dentistry. Page accessed August 16, 2006.
- ↑ FAQs About Root Canal Treatment Archived 2006-08-13 at the Wayback Machine, hosted by the American Association of Endodontists website. Page accessed August 16, 2006.
- ↑ Wisdom Teeth Archived 2006-08-23 at the Wayback Machine, a packet in pdf format hosted by the American Association of Oral and Maxillofacial Surgeons. Page accessed August 16, 2006.
- ↑ "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on 2009-11-11. Retrieved Nov 11, 2009.
- ↑ The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century – the approach of the WHO Global Oral Health Programme Archived 2006-09-27 at the Wayback Machine, released by the World Health Organization. (File in pdf format.) Page accessed August 15, 2006.
- ↑ Healthy People: 2010 Archived 2006-08-13 at the Wayback Machine. Html version hosted on Healthy People.gov Archived 2017-03-10 at the Wayback Machine website. Page accessed August 13, 2006.
- ↑ Frequently Asked Questions Archived 2006-08-16 at the Wayback Machine, hosted on the American Dental Hygiene Association website. Page accessed August 15, 2006.
- ↑ 148.0 148.1 148.2 "Dental caries Archived 2008-12-07 at the Wayback Machine", from the Disease Control Priorities Project. Page accessed August 15, 2006.
- ↑ "Archive copy" (PDF). Archived (PDF) from the original on 2018-07-04. Retrieved 2020-08-04.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help)CS1 maint: archived copy as title (link) - ↑ World Health Organization Archived 2006-03-26 at the Wayback Machine website, "World Water Day 2001: Oral health", p. 2. Page accessed August 14, 2006.
- ↑ 151.0 151.1 Touger-Decker R, van Loveren C (October 2003). "Sugars and dental caries". The American Journal of Clinical Nutrition. 78 (4): 881S–892S. doi:10.1093/ajcn/78.4.881S. PMID 14522753.
- ↑ "Table 38.1. Mean DMFT and SiC Index of 12-Year-Olds for Some Countries, by Ascending Order of DMFT Archived 2008-12-07 at the Wayback Machine", from the Disease Control Priorities Project. Page accessed January 8, 2007.
- ↑ Africa, Charlene; Nel, Janske; Stemmet, Megan (2014). "Anaerobes and Bacterial Vaginosis in Pregnancy: Virulence Factors Contributing to Vaginal Colonisation". International Journal of Environmental Research and Public Health. 11 (7): 6979–7000. doi:10.3390/ijerph110706979. ISSN 1660-4601. PMC 4113856. PMID 25014248.
- ↑ Towle, Ian; Irish, Joel D.; Groote, Isabelle De (2017). "Dental pathology, wear, and developmental defects in fossil hominins and extant primates". ResearchGate. Unpublished. doi:10.13140/RG.2.2.21395.99369. Archived from the original on 2020-05-09. Retrieved 2019-01-09.
{{cite journal}}: More than one of|accessdate=and|access-date=specified (help); More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ 155.0 155.1 155.2 Epidemiology of Dental Disease Archived 2006-11-29 at the Wayback Machine, hosted on the University of Illinois at Chicago website. Page accessed January 9, 2007.
- ↑ 156.0 156.1 156.2 156.3 156.4 156.5 156.6 156.7 156.8 Suddick RP, Harris NO (1990). "Historical perspectives of oral biology: a series". Critical Reviews in Oral Biology & Medicine. 1 (2): 135–51. doi:10.1177/10454411900010020301. PMID 2129621.
- ↑ Caries Through Time: An Anthropological Overview; Luis Pezo Lanfranco and Sabine Eggers; Laboratório de Antropologia Biológica, Depto. de Genética e Biologia Evolutiva, Instituto de Biociências, Universidade de São Paulo, Brazil
- ↑ Richards MP (December 2002). "A brief review of the archaeological evidence for Palaeolithic and Neolithic subsistence". European Journal of Clinical Nutrition. 56 (12): 1270–1278. doi:10.1038/sj.ejcn.1601646. PMID 12494313.
- ↑ Lukacs, John R. (1996-02-01). "Sex Differences in Dental Caries Rates With the Origin of Agriculture in South Asia". Current Anthropology. 37 (1): 147–153. doi:10.1086/204481. ISSN 0011-3204. Archived from the original on 2020-08-04. Retrieved 2020-08-04.
{{cite journal}}: More than one of|accessdate=and|access-date=specified (help); More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Tayles N.; Domett K.; Nelsen K. (2000). "Agriculture and dental caries? The case of rice in prehistoric Southeast Asia". World Archaeology. 32 (1): 68–83. doi:10.1080/004382400409899. PMID 16475298.
- ↑ 161.0 161.1 History of Dentistry: Ancient Origins Archived 2007-07-05 at the Wayback Machine, hosted on the American Dental Association Archived 2005-01-03 at the Wayback Machine website. Page accessed January 9, 2007.
- ↑ Coppa A, Bondioli L, Cucina A, et al. (April 2006). "Palaeontology: early Neolithic tradition of dentistry". Nature. 440 (7085): 755–6. Bibcode:2006Natur.440..755C. doi:10.1038/440755a. PMID 16598247. Lay summary.
{{cite journal}}: Cite uses deprecated parameter|lay-url=(help); Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help) - ↑ Anderson T (October 2004). "Dental treatment in Medieval England". British Dental Journal. 197 (7): 419–25. doi:10.1038/sj.bdj.4811723. PMID 15475905.
- ↑ Elliott, Jane (October 8, 2004). Medieval teeth 'better than Baldrick's' Archived 2007-01-11 at the Wayback Machine, BBC news.
- ↑ Gerabek WE (March 1999). "The tooth-worm: historical aspects of a popular medical belief". Clinical Oral Investigations. 3 (1): 1–6. doi:10.1007/s007840050070. PMID 10522185.
- ↑ McCauley, H. Berton. Pierre Fauchard (1678–1761) Archived 2007-04-04 at the Wayback Machine, hosted on the Pierre Fauchard Academy website. The excerpt comes from a speech given at a Maryland PFA Meeting on March 13, 2001. Page accessed January 17, 2007.
- ↑ Kleinberg I (1 March 2002). "A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis". Critical Reviews in Oral Biology & Medicine. 13 (2): 108–25. doi:10.1177/154411130201300202. PMID 12097354.
- ↑ Baehni PC, Guggenheim B (1996). "Potential of diagnostic microbiology for treatment and prognosis of dental caries and periodontal diseases" (PDF). Critical Reviews in Oral Biology & Medicine. 7 (3): 259–77. doi:10.1177/10454411960070030401. PMID 8909881. Archived from the original (PDF) on 2017-09-22. Retrieved 2020-08-04.
{{cite journal}}: More than one of|accessdate=and|access-date=specified (help); More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Hiremath, S. S. (2011). Textbook of Preventive and Community Dentistry. Elsevier India. p. 145. ISBN 9788131225301. Archived from the original on 2021-08-29. Retrieved 2020-08-04.
{{cite book}}: More than one of|accessdate=and|access-date=specified (help); More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Selwitz RH, Ismail AI, Pitts NB (January 2007). "Dental caries". The Lancet. 369 (9555): 51–9. doi:10.1016/S0140-6736(07)60031-2. PMID 17208642.
- ↑ Elsevier, Dorland's Illustrated Medical Dictionary, archived from the original on 2014-01-11, retrieved 2020-08-04.
{{citation}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Peneva, Milena (September 2008). "Activity of the caries process" (PDF). Ohdmbsc. VII (3): 3–8. Archived (PDF) from the original on 2019-09-26. Retrieved 2019-09-26.
{{cite journal}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ "Dentistry for All / International Dental Review – General Info". Archived from the original on 2019-10-26. Retrieved 2019-09-26.
...Head of Cariesology and Endodontics Department...
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ "The Faculty of Dental Medicine". Archived from the original on 2019-09-26. Retrieved 2019-09-26.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ ""Cariology" entry". thefreedictionary.com. Archived from the original on 2019-09-26. Retrieved 2019-09-26.
{{cite web}}: More than one of|archivedate=and|archive-date=specified (help); More than one of|archiveurl=and|archive-url=specified (help) - ↑ Listl, S.; Galloway, J.; Mossey, P. A.; Marcenes, W. (28 August 2015). "Global Economic Impact of Dental Diseases". Journal of Dental Research. 94 (10): 1355–1361. doi:10.1177/0022034515602879. PMID 26318590.
- ↑ "AVDS Cavities information page — Dog Tooth Health — Cat Tooth Health". American Veterinary Dental Society. Archived from the original on 13 October 2006. Retrieved 10 November 2013.
Cited sources
- Nanci, A. (2013). Ten Cate's Oral Histology. Elsevier. ISBN 978-0323078467.
External links
| Classification | |
|---|---|
| External resources |
- Tooth decay at Curlie
- Centers for Disease Control: Dental Caries Archived 2020-08-04 at the Wayback Machine
- Pages with script errors
- CS1 errors: redundant parameter
- Webarchive template wayback links
- CS1: long volume value
- CS1 maint: archived copy as title
- CS1 maint: multiple names: authors list
- CS1 maint: others
- Wikipedia articles needing page number citations from August 2014
- Articles with invalid date parameter in template
- CS1 maint: location
- CS1 errors: unsupported parameter
- CS1 errors: deprecated parameters
- Articles with hatnote templates targeting a nonexistent page
- All articles with unsourced statements
- Articles with unsourced statements from February 2020
- Articles needing additional references from November 2016
- All articles needing additional references
- Articles with unsourced statements from September 2018
- Articles with medical app sidebar
- Articles with Curlie links
- Acquired tooth disorders
- Dental caries
- RTT
- Acute pain
