Complex regional pain syndrome
| Complex regional pain syndrome | |
|---|---|
| Other names: Post-traumatic pain syndrome,[1] reflex sympathetic dystrophy (RSD), causalgia,[2] reflex neurovascular dystrophy (RND) | |
 | |
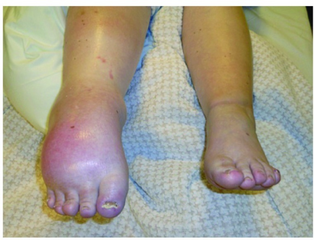
| Left leg affected by complex regional pain syndrome following a tibial bone fracture. | |
| Specialty | Neurology, anesthesiology |
| Symptoms | Region of pain, swelling, skin changes, limited range of motion, temperatures changes[3] |
| Duration | Short or long-term[2] |
| Types | Type I, type II[3] |
| Causes | Following tissue injury[3] |
| Risk factors | Psychological stress[3] |
| Diagnostic method | Based on symptoms after ruling out other potential causes[3] |
| Differential diagnosis | Neuropathy, cellulitis, erythromelalgia, vasculitis, chronic venous insufficiency, lymphedema, deep vein thrombosis, Raynaud’s, porphyria[3] |
| Treatment | Physiotherapy, medications, cognitive behavioral therapy[3] |
| Prognosis | Variable[2] |
| Frequency | 6 per 100,000 people per year[3] |
Complex regional pain syndrome (CRPS) is a condition characterized by prolonged and excessive pain and inflammation following an injury.[2] Other symptoms often include swelling, skin changes, limited range of motion, and temperatures changes in the affected area.[3] Most commonly it starts in a limb though may spread to other areas.[3]
It is unclear why CRPS occurs.[2] The trigger is typically some type of tissue injury, most commonly a bone fracture.[3] Risk factors include psychological stress.[3] The underlying mechanism involves abnormal neuronal transmission, autonomic dysregulation, and central sensitization.[3] It is a type of neuropathic pain of which there are two subtypes.[3] Diagnosis is based on symptoms after ruling out other potential causes.[3]
Evidence for specific treatments is overall poor.[4] Different measures may include physiotherapy, cognitive behavioral therapy, steroids, gabapentin, duloxetine, lidocaine patches, opioids, ketamine, bisphosphonates, and nerve blocks.[3][4] Components of physiotherapy may include graded motor imagery and mirror therapy.[4] Outcomes are variable.[2]
CRPS newly affects about 6 per 100,000 people per year.[3] Women are three times more commonly affected than men.[3] Onset is often around the age of 40.[2] The condition was first described in 1864 by Silas Weir Mitchell.[5]
Signs and symptoms
Symptoms of CRPS usually manifest near the injury site. The most common symptoms are extreme pain, including burning, stabbing, grinding, and throbbing. The pain is out of proportion to the severity of the initial injury.[6] Moving or touching the limb is often intolerable. With diagnosis of either CRPS types I or II, patients may develop burning pain and allodynia (pain to non-painful stimuli). Both syndromes are also characterized by autonomic dysfunction, which presents with localized temperature changes, cyanosis, and/or edema.[citation needed]
People may also experience localized swelling; extreme sensitivity to nonpainful stimuli such as wind, water, noise, and vibrations; extreme sensitivity to touch (by themselves, other people, and even their clothing or bedding/blankets); abnormally increased sweating (or absent sweating); changes in skin temperature (alternating between sweaty and cold); changes in skin colouring (from white and mottled to bright red or reddish-violet); changes in skin texture (waxy, shiny, thin, tight skin); softening and thinning of bones; joint tenderness or stiffness; changes in nails and hair (delayed or increased growth, brittle nails/hair that easily break); muscle spasms; muscle loss (atrophy); tremors; dystonia; allodynia; hyperalgesia; and decreased/restricted ability and painful movement of affected body part.[6] Drop attacks (falls), almost fainting, and fainting spells are infrequently reported, as are visual problems.[citation needed]
The symptoms of CRPS vary in severity and duration. One version of the McGill pain index, a scale for rating pain, ranks CRPS highest, above childbirth, amputation and cancer.[7] Since CRPS is a systemic problem, potentially any organ can be affected. Symptoms may change over time, and they can vary from person to person. Symptoms can even change numerous times in a single day.[citation needed]
Previously, CRPS was considered to have three stages however more recent studies suggest people affected by CRPS do not progress through sequential stages and the staging system is no longer in wide use.[8] Growing evidence instead points towards distinct sub-types of CRPS.[8]
-
CRPS-1 (Complex regional pain syndrome): dystonic equinus of the right ankle, swollen foot and calf with tightened, pale, gleaming and cold skin.
-
Severe CRPS of right arm
-
CRPS visible on hands and wrists
Cause
Complex regional pain syndrome is uncommon, and its cause is not clearly understood. CRPS typically develops after an injury, surgery, heart attack, or stroke.[6][9] Investigators estimate that 2–5% of those with peripheral nerve injury,[10] and 13-70% of those with hemiplegia (paralysis of one side of the body)[11] will develop CRPS. In addition, some studies have indicated that cigarette smoking was strikingly present in patients and is statistically linked to RSD. This may be involved in its pathology by enhancing sympathetic activity, vasoconstriction, or by some other unknown neurotransmitter-related mechanism. This hypothesis was based on a retrospective analysis of 53 patients with RSD, which showed that 68% of patients and only 37% of controls were smokers. The results are preliminary and are limited by their retrospective nature.[12] 7% of people who have CRPS in one limb later develop it in another limb.[13]
Pathophysiology

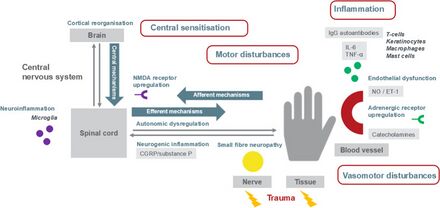
The underlying mechanism is beleived to involve inflammation resulting from the release of certain proinflammatory chemical signals from the nerves, sensitized nerve receptors that send pain signals to the brain, dysfunction of the local blood vessels' ability to constrict and dilate appropriately, and maladaptive neuroplasticity.[16]
Inflammation and alteration of pain perception in the central nervous system are proposed to play important roles. The persistent pain and the perception of nonpainful stimuli as painful are thought to be caused by inflammatory molecules (IL-1, IL2, TNF-alpha) and neuropeptides (substance P) released from peripheral nerves. This release may be caused by inappropriate cross-talk between sensory and motor fibers at the affected site.[17] CRPS is not a psychological illness, yet pain can cause psychological problems, such as anxiety and depression. Often, impaired social and occupational function occur.[18]
Complex regional pain syndrome is a multifactorial disorder with clinical features of neurogenic inflammation (swelling in the central nervous system), nociceptive sensitisation (which causes extreme sensitivity or allodynia), vasomotor dysfunction (blood flow problems which cause swelling and discolouration) and maladaptive neuroplasticity (where the brain changes and adapts with constant pain signals); CRPS is the result of an "aberrant [inappropriate] response to tissue injury".[16] The "underlying neuronal matrix" of CRPS is seen to involve cognitive and motor as well as nociceptive processing; pinprick stimulation of a CRPS affected limb was painful (mechanical hyperalgesia) and showed a "significantly increased activation" of not just the S1 cortex (contralateral), S2 (bilateral) areas, and insula (bilateral) but also the associative-somatosensory cortices (contralateral), frontal cortices, and parts of the anterior cingulate cortex.[19] In contrast to previous thoughts reflected in the name RSD, it appears that there is reduced sympathetic nervous system outflow, at least in the affected region (although there may be sympatho-afferent coupling).[20] Wind-up (the increased sensation of pain with time)[21] and central nervous system (CNS) sensitization are key neurologic processes that appear to be involved in the induction and maintenance of CRPS.[22]
Compelling evidence shows that the N-methyl-D-aspartate (NMDA) receptor has significant involvement in the CNS sensitization process.[23] It is also hypothesized that elevated CNS glutamate levels promote wind-up and CNS sensitization.[22] In addition, there exists experimental evidence demonstrating the presence of NMDA receptors in peripheral nerves.[24] Because immunological functions can modulate CNS physiology, a variety of immune processes have also been hypothesized to contribute to the initial development and maintenance of peripheral and central sensitization.[25][26] Furthermore, trauma related cytokine release, exaggerated neurogenic inflammation, sympathetic afferent coupling, adrenoreceptor pathology, glial cell activation, cortical reorganisation,[27] and oxidative damage (e.g., by free radicals) are all factors which have been implicated in the pathophysiology of CRPS.[28] In addition, autoantibodies are present in a wide number of CRPS patients and IgG has been recognized as one of the causes of hypersensitivity that stimulates A and C nociceptors, attributing to the inflammation.[29]
The mechanisms leading to reduced bone mineral density (up to overt osteoporosis) are still unknown. Potential explanations include a dysbalance of the activities of sympathetic and parasympathetic autonomic nervous system [30][31][32] and mild secondary hyperparathyroidism.[33] However, the trigger of secondary hyperparathyroidism has not yet been identified.[citation needed]
In summary, the pathophysiology of complex regional pain syndrome has not yet been defined; CRPS, with its variable manifestations, could be the result of multiple pathophysiological processes.[20]
Diagnosis

Diagnosis is primarily based on clinical findings. The original diagnostic criteria for CRPS adopted by the International Association for the Study of Pain (IASP) in 1994 have now been superseded in both clinical practice and research by the ‘‘Budapest Criteria” which were created in 2003 and have been found to be more sensitive and specific.[35] They have since been adopted by the IASP. The criteria require there to be pain as well as a history and clinical evidence of sensory, vasomotor, sudomotor and motor or trophic changes. It is also a diagnosis of exclusion.[36]
To make a clinical diagnosis all four of the following criteria must be met:[citation needed]
- Continuing pain, which is disproportionate to any inciting event
- Must report at least one symptom in three of the four following categories..
- Sensory: Reports of hyperesthesia
- Vasomotor: Reports of temperature asymmetry and/or skin color changes and/or skin color asymmetry
- Sudomotor/Edema: Reports of edema and/or sweating changes and/or sweating asymmetry
- Motor/Trophic: Reports of decreased range of motion and/or motor dysfunction (weakness, tremor, dystonia) and/or trophic changes (hair, nail, skin)
- Must display at least one sign at time of evaluation in two or more of the following categories
- Sensory: Evidence of hyperalgesia (to pinprick) and/or allodynia (to light touch and/or temperature sensation and/or deep somatic pressure and/or joint movement)
- Vasomotor: Evidence of temperature asymmetry (>1 °C) and/or skin color changes and/or asymmetry
- Sudomotor/Edema: Evidence of edema and/or sweating changes and/or sweating asymmetry
- Motor/Trophic: Evidence of decreased range of motion and/or motor dysfunction (weakness, tremor, dystonia) and/or trophic changes (hair, nail, skin)
- There is no other diagnosis that better explains the signs and symptoms
Thermography
Presently, established empirical evidence suggests against thermography's efficacy as a reliable tool for diagnosing CRPS. Although CRPS may, in some cases, lead to measurably altered blood flow throughout an affected region, many other factors can also contribute to an altered thermographic reading, including the patient's smoking habits, use of certain skin lotions, recent physical activity, and prior history of trauma to the region. Also, not all patients diagnosed with CRPS demonstrate such "vasomotor instability" — particularly those in the later stages of the disease.[37] Thus, thermography alone cannot be used as conclusive evidence for—or against—a diagnosis of CRPS and must be interpreted in light of the patient's larger medical history and prior diagnostic studies.[38]
Medical imaging
Plain X-rays, and magnetic resonance imaging may all be useful diagnostically. Patchy osteoporosis (post-traumatic osteoporosis), which may be due to disuse of the affected extremity, can be detected through X-ray imagery as early as two weeks after the onset of CRPS. A bone scan of the affected limb may detect these changes even sooner and can almost confirm the disease. Bone densitometry can also be used to detect changes in bone mineral density. It can also be used to monitor the results of treatment since bone densitometry parameters improve with treatment.[citation needed]
Ultrasound-based osteodensitometry (ultrasonometry) may be potential future radiation-free technique to identify reduced bone mineral density in CRPS.[33] Additionally, this method promises to quantify the bone architecture in the periphery of affected limbs.[33] This method is still under experimental development.[citation needed]
EMG
Electromyography (EMG) and nerve conduction studies (NCS) are important ancillary tests in CRPS because they are among the most reliable methods of detecting nerve injury. They can be used as one of the primary methods to distinguish between CRPS types I and II, which differ based on evidence of actual nerve damage. EMG and NCS are also among the best tests for ruling in or out alternative diagnoses. CRPS is a "diagnosis of exclusion", which requires that no other diagnosis can explain the patient's symptoms. This is very important to emphasise because patients otherwise can be given a wrong diagnosis of CRPS when they actually have a treatable condition that better accounts for their symptoms. An example is severe carpal tunnel syndrome (CTS), which can often present in a very similar way to CRPS. Unlike CRPS, CTS can often be corrected with surgery to alleviate the pain and avoid permanent nerve damage and malformation.[39]
Classification
The classification system currently in use by the International Association for the Study of Pain (IASP) divides CRPS into two types. It is recognised that people may exhibit both types of CRPS.[citation needed]
| Type | Clinical findings | Synonyms |
|---|---|---|
| Type I | CRPS without evidence of nerve damage in the affected limb. Secondary to injury/trauma. This accounts for about 90% of CRPS. | RSD, Sudeck's atrophy |
| Type II | CRPS with evidence of nerve damage in the affected limb. | Causalgia |
- Type I, formerly known as reflex sympathetic dystrophy (RSD), Sudeck's atrophy, or algoneurodystrophy, does not exhibit demonstrable nerve lesions. As the vast majority of patients diagnosed with CRPS have this type, it is most commonly referred to in medical literature as type I.[citation needed]
- Type II, formerly known as causalgia, has evidence of obvious nerve damage. Despite evidence of nerve injury, the cause or the mechanisms of CRPS type II are as unknown, as the mechanisms of type I.[citation needed]
Patients are frequently classified into two groups based upon temperature: "warm" or "hot" CRPS in one group and "cold" CRPS in the other group. The majority of patients (about 70%) have the "hot" type, which is said to be an acute form of CRPS.[40] Cold CRPS is said to be indicative of a more chronic CRPS and is associated with poorer McGill Pain Questionnaire scores, increased central nervous system involvement, and a higher prevalence of dystonia.[40] Prognosis is not favourable for cold CRPS patients; longitudinal studies suggest these patients have "poorer clinical pain outcomes and show persistent signs of central sensitisation correlating with disease progression".[41]
Prevention
Vitamin C may be useful in prevention of the syndrome following fracture of the forearm or foot and ankle.[42]
Treatment
Treatment of CRPS often involves a number of modalities.[43]
Therapy
Physical and occupational therapy have low-quality evidence to support their use.[4] Physical therapy interventions may include transcutaneous electrical nerve stimulation, progressive weight bearing, graded tactile desensitization, massage, and contrast bath therapy. In a retrospective cohort (unblinded, non-randomised and with intention-to-treat) of fifty patients diagnosed with CRPS, the subjective pain and body perception scores of patients decreased after engagement with a two-week multidisciplinary rehabilitation programme. The authors call for randomised controlled trials to probe the true value of multidisciplinary programs for CRPS patients.[44]
Mirror box
Mirror box therapy uses a mirror box, or a stand-alone mirror, to create a reflection of the normal limb such that the patient thinks they are looking at the affected limb. Movement of this reflected normal limb is then performed so that it looks to the patient as though they are performing movement with the affected limb. Mirror box therapy appears to be beneficial at least in early CRPS.[4] However, beneficial effects of mirror therapy in the long term is still unproven.[45]
Graded motor imagery
Graded motor imagery appears to be useful for people with CRPS-1.[46] Graded motor imagery is a sequential process that consists of (a) laterality reconstruction, (b) motor imagery, and (c) mirror therapy.[43][47]
Transcutaneous nerve stimulation
Transcutaneous electrical nerve stimulation(TENS) is a therapy that uses low voltage electrical signals to provide pain relief through electrodes that are placed on the surface of the skin. Evidence supports its use in treating pain and edema associated with CRPS although it doesn’t seem to increase functional ability in CRPS patients.[48]
Medications
Tentative evidence supports the use of bisphosphonates, calcitonin, and ketamine.[4] Nerve blocks with guanethidine appear to be harmful.[4] Evidence for sympathetic nerve blocks generally is insufficient to support their use.[49] Intramuscular botulinum injections may benefit people with symptoms localized to one extremity.[50]
Ketamine
Ketamine, a dissociative anesthetic, appears promising as a treatment for CRPS.[51] It may be used in low doses if other treatments have not worked.[52][53] No benefit on either function or depression, however, has been seen.[53]
Bisphosphonates
As of 2013, low-quality evidence supports the use of bisphosphonates.[4] A 2009 review found "very limited data reviewed showed that bisphosphonates have the potential to reduce pain associated with bone loss in patients with CRPS I, however, at present evidence is not sufficient to recommend their use in practice".[54]
Opioids
Opioids such as oxycodone, morphine, hydrocodone, and fentanyl have a controversial place in treatment of CRPS. These drugs must be prescribed and monitored under close supervision of a physician, as these drugs will lead to physical dependence and can lead to addiction.[55] Thus far, no long-term studies of oral opioid use in treating neuropathic pain, including CRPS, have been performed. The consensus among experts is that opioids should not be a first line therapy and should only be considered after all other modalities (non-opioid medications, physical therapy, and procedures) have been trialed.[56]
Surgery
Spinal cord stimulators
Spinal cord stimulator appears to be an effective therapy in the management of patients with CRPS type I (level A evidence) and type II (level D evidence).[57] While they improve pain and quality of life, evidence is unclear regarding effects on mental health and general functioning.[58]
Dorsal root ganglion stimulation is type of neurostimulation that is effective in the management of focal neuropathic pain. The FDA approved its use in February 2016. The ACCURATE Study demonstrated superiority of dorsal root ganglion stimulation over spinal (dorsal column) stimulation in the management of CRPS and causalgia.[59]
Sympathectomy
Surgical, chemical, or radiofrequency sympathectomy — interruption of the affected portion of the sympathetic nervous system — can be used as a last resort in patients with impending tissue loss, edema, recurrent infection, or ischemic necrosis.[60] However, little evidence supports these permanent interventions to alter the pain symptoms of the affected patients, and in addition to the normal risks of surgery, such as bleeding and infection, sympathectomy has several specific risks, such as adverse changes in how nerves function.[citation needed]
Amputation
No randomized study in medical literature has studied the response with amputation of patients who have failed the above-mentioned therapies and who continue to be miserable. Nonetheless, on average, about half of the patients will have resolution of their pain, while half will develop phantom limb pain and/or pain at the amputation site. As in any other chronic pain syndrome, the brain likely becomes chronically stimulated with pain, and late amputation may not work as well as it might be expected. In a survey of 15 patients with CRPS type 1, 11 responded that their lives were better after amputation.[61] Since this is the ultimate treatment of a painful extremity, it should be left as a last resort.[citation needed]
Prognosis
Good progress can be made in treating CRPS if treatment is begun early, ideally within three months of the first symptoms. If treatment is delayed, however, the disorder can quickly spread to the entire limb, and changes in bone, nerve, and muscle may become irreversible. The prognosis is not always good. Johns Hopkins Hospital reports that 77% of sufferers have spreads from the original site or flares in other parts of the body. The limb, or limbs, can experience muscle atrophy, loss of use, and functionally useless parameters that require amputation. RSD/CRPS will not "burn itself out", but if treated early, it is likely to be manageable. Once one is diagnosed with CRPS, should it go into remission, the likelihood of it resurfacing after going into remission is significant. Taking precautions and seeking immediate treatment upon any injury is important.[62]
Epidemiology
CRPS can occur at any age, with the average age at diagnosis being 42.[10] It affects both men and women; however, CRPS is three times more frequent in females than males.[10]
CRPS affects both adults and children, and the number of reported CRPS cases among adolescents and young adults has been increasing,[63] with a recent observational study finding an incidence of 1.16/100,000 among children in Scotland.[64]
History
The condition currently known as CRPS was originally described during the American Civil War by Silas Weir Mitchell, who is sometimes also credited with inventing the name "causalgia".[65] However, this term was actually coined by Mitchell's friend Robley Dunglison from the Greek words for heat and for pain.[66] Contrary to what is commonly accepted, it emerges that these causalgias were certainly major by the importance of the vasomotor and sudomotor symptoms but stemmed from minor neurological lesions. In the 1940s, the term reflex sympathetic dystrophy came into use to describe this condition, based on the theory that sympathetic hyperactivity was involved in the pathophysiology.[67] In 1959, Noordenbos observed in causalgia patients that "the damage of the nerve is always partial."[68] Misuse of the terms, as well as doubts about the underlying pathophysiology, led to calls for better nomenclature. In 1993, a special consensus workshop held in Orlando, Florida, provided the umbrella term "complex regional pain syndrome", with causalgia and RSD as subtypes.[69]
Research
The National Institute of Neurological Disorders and Stroke (NINDS), a part of the National Institutes of Health, supports and conducts research on the brain and central nervous system, including research relevant to RSDS, through grants to major medical institutions across the country. NINDS-supported scientists are working to develop effective treatments for neurological conditions and ultimately, to find ways of preventing them. Investigators are studying new approaches to treat CRPS and intervene more aggressively after traumatic injury to lower the patient's chances of developing the disorder. In addition, NINDS-supported scientists are studying how signals of the sympathetic nervous system cause pain in CRPS patients. Using a technique called microneurography, these investigators are able to record and measure neural activity in single nerve fibers of affected patients. By testing various hypotheses, these researchers hope to discover the unique mechanism that causes the spontaneous pain of CRPS, and that discovery may lead to new ways of blocking pain. Other studies to overcome chronic pain syndromes are discussed in the pamphlet "Chronic Pain: Hope Through Research", published by the NINDS.[citation needed]
Research into treating the condition with mirror visual feedback is being undertaken at the Royal National Hospital for Rheumatic Disease in Bath. Patients are taught how to desensitize in the most effective way, then progress to using mirrors to rewrite the faulty signals in the brain that appear responsible for this condition.[70] However, while CRPS can go into remission, the chance of it reoccurring is significant.[citation needed]
The Netherlands currently has the most comprehensive program of research into CRPS, as part of a multimillion-Euro initiative called TREND.[71] German and Australian research teams are also pursuing better understanding and treatments for CRPS.[citation needed]
Society and culture
Notable cases
- Nia Frazier, Dance Moms star[72]
- Paula Abdul, singer, actor, TV personality[73]
- Jill Kinmont Boothe, US ski slalom champion[74]
- Gemma Collis, British paralympic fencer[75]
- Shin Dong-wook, South Korean actor and model[76]
- Howard Hughes, American business tycoon, aviator, inventor, filmmaker, and philanthropist[77]
- Rachel Morris, British paralympic cyclist.[78]
- Cynthia Toussaint, author and media personality[79]
- Danielle Brown, British paralympic archer[80]
- Radene Marie Cook, former Los Angeles radio broadcaster, artist, and advocate[81]
- Marieke Vervoort, Belgian Paralympic athlete[82]
- Bruno Soriano, Spanish footballer[83]
Other animals
CRPS has also been described in animals, such as cattle.[84]
References
- ↑ Aburahma, Ali F.; Mousa, Albeir Y. (30 August 2012). "Post‐Traumatic Pain Syndrome: Complex Regional Pain Syndrome". Haimovici's Vascular Surgery: 932–946. doi:10.1002/9781118481370.ch74. Archived from the original on 20 September 2024. Retrieved 16 September 2024.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Complex Regional Pain Syndrome Fact Sheet | National Institute of Neurological Disorders and Stroke". www.ninds.nih.gov. Archived from the original on 1 March 2021. Retrieved 9 March 2021.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 Guthmiller, KB; Varacallo, M (January 2021). "Complex Regional Pain Syndrome". StatPearls. PMID 28613470.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 O'Connell, NE; Wand, BM; McAuley, J; Marston, L; Moseley, GL (30 April 2013). "Interventions for treating pain and disability in adults with complex regional pain syndrome". The Cochrane database of systematic reviews (4): CD009416. doi:10.1002/14651858.CD009416.pub2. PMID 23633371.
- ↑ Buschbacher, Ralph M. (2002). Practical Guide to Musculoskeletal Disorders: Diagnosis and Rehabilitation. Butterworth-Heinemann. p. 269. ISBN 978-0-7506-7357-0. Archived from the original on 2021-08-28. Retrieved 2021-03-09.
- ↑ 6.0 6.1 6.2 "Complex regional pain syndrome - Symptoms and causes". Mayo Clinic. Archived from the original on 2018-07-24. Retrieved 2018-07-24.
- ↑ "McGILL Pain Index and CRPS or RSD". Archived from the original on 2012-11-09. Retrieved 2012-12-05.
- ↑ 8.0 8.1 Bruehl, Stephen; Harden, R. Norman; Galer, Bradley S.; Saltz, Samuel; Backonja, Miroslav; Stanton-Hicks, Michael (January 2002). "Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome?". Pain. 95 (1–2): 119–124. doi:10.1016/s0304-3959(01)00387-6. ISSN 0304-3959. PMID 11790474. S2CID 20773804.
- ↑ "Complex regional pain syndrome". nhs.uk. 2017-10-19. Archived from the original on 2016-08-26. Retrieved 2016-08-25.
- ↑ 10.0 10.1 10.2 Veldman PH, Reynen HM, Arntz IE, Goris RJ (October 1993). "Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients". Lancet. 342 (8878): 1012–6. doi:10.1016/0140-6736(93)92877-V. PMID 8105263. S2CID 39843988.
- ↑ Yu D (August 2004). "Shoulder pain in hemiplegia". Physical Medicine and Rehabilitation Clinics of North America. 15 (3): vi–vii, 683–97. doi:10.1016/S1047-9651(03)00130-X. PMID 15219895.
- ↑ Pawelka S, Fialka V, Ernst E (January 1993). "Reflex sympathetic dystrophy and cigarette smoking". The Journal of Hand Surgery. 18 (1): 168–9. doi:10.1016/0363-5023(93)90273-6. PMID 8423309.
- ↑ Complex regional pain syndrome in adults - UK guidelines for diagnosis, referral and management in primary and secondary care; Royal College of Physicians London (May 2012)
- ↑ Alshehri, Fahad S. (October 2023). "The complex regional pain syndrome: Diagnosis and management strategies". Neurosciences. 28 (4): 211. doi:10.17712/nsj.2023.4.20230034. Retrieved 19 June 2024.
- ↑ Mangnus, Thomas J. P.; Bharwani, Krishna D.; Dirckx, Maaike; Huygen, Frank J. P. M. (April 2022). "From a Symptom-Based to a Mechanism-Based Pharmacotherapeutic Treatment in Complex Regional Pain Syndrome". Drugs. 82 (5): 511–531. doi:10.1007/s40265-022-01685-4. ISSN 1179-1950.
- ↑ 16.0 16.1 Marinus J, Moseley GL, Birklein F, et al. (July 2011). "Clinical features and pathophysiology of complex regional pain syndrome". Lancet Neurology. 10 (7): 637–48. doi:10.1016/S1474-4422(11)70106-5. PMC 5511749. PMID 21683929.
- ↑ Complex regional pain syndrome type I: a comprehensive review. Bussa M, Guttilla D, Lucia M, Mascaro A, Rinaldi S. Acta Anaesthesiol Scand. 2015 Jul;59(6):685-97. Epub 2015 Apr 22.
- ↑ Lohnberg JA, Altmaier EM (June 2013). "A review of psychosocial factors in complex regional pain syndrome". Journal of Clinical Psychology in Medical Settings. 20 (2): 247–54. doi:10.1007/s10880-012-9322-3. PMID 22961122. S2CID 14756892.
- ↑ Maihöfner C, Forster C, Birklein F, Neundörfer B, Handwerker HO (March 2005). "Brain processing during mechanical hyperalgesia in complex regional pain syndrome: a functional MRI study". Pain. 114 (1–2): 93–103. doi:10.1016/j.pain.2004.12.001. PMID 15733635. S2CID 19187294.
- ↑ 20.0 20.1 DR WILL HOWARD FFPMANZCA, FFANZCA, DIP MED (PAIN MANAGEMENT) http://www.qld.anzca.edu.au/anzca/resources/college-publications/pdfs/ANZCA%20Blue%20Book%202011%20P9.pdf#page=10 Archived 2013-09-21 at the Wayback Machine[full citation needed]
- ↑ "Pain". Courses.washington.edu. Archived from the original on 2012-02-03. Retrieved 2013-12-23.
- ↑ 22.0 22.1 Correll GE, Maleki J, Gracely EJ, Muir JJ, Harbut RE (September 2004). "Subanesthetic ketamine infusion therapy: a retrospective analysis of a novel therapeutic approach to complex regional pain syndrome". Pain Medicine. 5 (3): 263–75. doi:10.1111/j.1526-4637.2004.04043.x. PMID 15367304.
- ↑ Kiefer RT, Rohr P, Ploppa A, et al. (2008). "A pilot open-label study of the efficacy of subanesthetic isomeric S(+)-ketamine in refractory CRPS patients". Pain Med. 9 (1): 44–54. doi:10.1111/j.1526-4637.2006.00223.x. PMID 18254766.
- ↑ Pöyhiä R, Vainio A (January 2006). "Topically administered ketamine reduces capsaicin-evoked mechanical hyperalgesia". The Clinical Journal of Pain. 22 (1): 32–6. doi:10.1097/01.ajp.0000149800.39240.95. PMID 16340591. S2CID 14035667.
- ↑ Watkins LR, Maier SF (February 2005). "Immune regulation of central nervous system functions: from sickness responses to pathological pain". Journal of Internal Medicine. 257 (2): 139–55. doi:10.1111/j.1365-2796.2004.01443.x. PMID 15656873. S2CID 24853745.
- ↑ Koffler SP, Hampstead BM, Irani F, et al. (August 2007). "The neurocognitive effects of 5-day anesthetic ketamine for the treatment of refractory complex regional pain syndrome". Archives of Clinical Neuropsychology. 22 (6): 719–29. doi:10.1016/j.acn.2007.05.005. PMID 17611073.
- ↑ Birklein F (February 2005). "Complex regional pain syndrome". Journal of Neurology. 252 (2): 131–8. CiteSeerX 10.1.1.483.1324. doi:10.1007/s00415-005-0737-8. PMID 15729516. S2CID 2965351.
- ↑ Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW (July 2007). "Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study". The Journal of Bone and Joint Surgery. American Volume. 89 (7): 1424–31. doi:10.2106/JBJS.F.01147. PMID 17606778.
- ↑ Cuhadar U, Gentry C, Vastani N, Sensi S, Bevan S, Goebel A, Andersson DA, et al. (2019). "Autoantibodies produce pain in complex regional pain syndrome by sensitizing nociceptors". Pain Med. 160 (12): 2855–2865. doi:10.1097/j.pain.0000000000001662. PMID 31343542.
- ↑ Bajayo, A; Bar, A; Denes, A; Bachar, M; Kram, V; Attar-Namdar, M; Zallone, A; Kovács, KJ; Yirmiya, R; Bab, I (18 September 2012). "Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual". Proceedings of the National Academy of Sciences of the United States of America. 109 (38): 15455–60. Bibcode:2012PNAS..10915455B. doi:10.1073/pnas.1206061109. PMC 3458367. PMID 22949675.
- ↑ Kondo, H; Takeuchi, S; Togari, A (1 March 2013). "β-Adrenergic signaling stimulates osteoclastogenesis via reactive oxygen species". American Journal of Physiology. Endocrinology and Metabolism. 304 (5): E507-15. doi:10.1152/ajpendo.00191.2012. PMID 23169789.
- ↑ Nencini, S; Ivanusic, JJ (2016). "The Physiology of Bone Pain. How Much Do We Really Know?". Frontiers in Physiology. 7: 157. doi:10.3389/fphys.2016.00157. PMC 4844598. PMID 27199772.
- ↑ 33.0 33.1 33.2 Bazika-Gerasch, B; Maier, C; Kumowski, N; Fiege, C; Kaisler, M; Vollert, J; Dietrich, JW (June 2019). "Compared to limb pain of other origin, ultrasonographic osteodensitometry reveals loss of bone density in complex regional pain syndrome". Pain. 160 (6): 1261–1269. doi:10.1097/j.pain.0000000000001520. PMID 30747906.
- ↑ Crock, Lara W.; Baldridge, Megan T. (1 August 2020). "A role for the microbiota in complex regional pain syndrome?". Neurobiology of Pain. 8: 100054. doi:10.1016/j.ynpai.2020.100054. ISSN 2452-073X.
- ↑ Harden, Norman R.; Bruehl, Stephen; Perez, Roberto S.G.M.; Birklein, Frank; Marinus, Johan; Maihofner, Christian; Lubenow, Timothy; Buvanendran, Asokumar; Mackey, Sean (August 2010). "Validation of proposed diagnostic criteria (the "Budapest Criteria") for Complex Regional Pain Syndrome". Pain. 150 (2): 268–274. doi:10.1016/j.pain.2010.04.030. ISSN 0304-3959. PMC 2914601. PMID 20493633.
- ↑ Frontera, Walter R.; Silver, Julie K.; Rizzo, Thomas D. (2014-09-05). Essentials of Physical Medicine and Rehabilitation E-Book. Elsevier Health Sciences. ISBN 978-0-323-22272-3. Archived from the original on 2021-08-28. Retrieved 2020-12-24.
- ↑ Birklein F, Künzel W, Sieweke N (August 2001). "Despite clinical similarities there are significant differences between acute limb trauma and complex regional pain syndrome I (CRPS I)". Pain. 93 (2): 165–71. doi:10.1016/s0304-3959(01)00309-8. PMID 11427328. S2CID 20172363.
- ↑ Wasner G, Schattschneider J, Baron R (July 2002). "Skin temperature side differences--a diagnostic tool for CRPS?". Pain. 98 (1–2): 19–26. doi:10.1016/s0304-3959(01)00470-5. PMID 12098613. S2CID 24474769.
- ↑ "Reflex Sympathetic Dystrophy Clinical Practice Guidelines". Rsdfoundation.org. 2003-01-01. Archived from the original on 2013-11-02. Retrieved 2013-12-23.
- ↑ 40.0 40.1 Eberle T, Doganci B, Krämer HH, et al. (February 2009). "Warm and cold complex regional pain syndromes: differences beyond skin temperature?". Neurology. 72 (6): 505–12. doi:10.1212/01.wnl.0000341930.35494.66. PMID 19204260. S2CID 6670243.
- ↑ Vaneker M, Wilder-Smith OH, Schrombges P, de Man-Hermsen I, Oerlemans HM (May 2005). "Patients initially diagnosed as "warm" or "cold" CRPS 1 show differences in central sensory processing some eight years after diagnosis: a quantitative sensory testing study". Pain. 115 (1–2): 204–11. doi:10.1016/j.pain.2005.02.031. PMID 15836983. S2CID 11529390.
- ↑ Meena, S; Sharma, P; Gangary, SK; Chowdhury, B (9 December 2014). "Role of vitamin C in prevention of complex regional pain syndrome after distal radius fractures: a meta-analysis". European Journal of Orthopaedic Surgery & Traumatology : Orthopedie Traumatologie. 25 (4): 637–41. doi:10.1007/s00590-014-1573-2. PMID 25488053. S2CID 22016034.
- ↑ 43.0 43.1 Shah A, Kirchner JS (June 2011). "Complex regional pain syndrome". Foot and Ankle Clinics. 16 (2): 351–66. CiteSeerX 10.1.1.483.1324. doi:10.1016/j.fcl.2011.03.001. PMID 21600455.
- ↑ Lewis, Jennifer S.; Kellett, Sara; McCullough, Ryan; Tapper, Ashley; Tyler, Chelsey; Viner, Maria; Palmer, Shea (2019-08-02). "Body Perception Disturbance and Pain Reduction in Longstanding Complex Regional Pain Syndrome Following a Multidisciplinary Rehabilitation Program". Pain Medicine (Malden, Mass.). 20 (11): 2213–2219. doi:10.1093/pm/pnz176. ISSN 1526-4637. PMID 31373373.
- ↑ Moseley, G; Zalucki, N; Wiech, K (2008). "Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain". Pain. 137 (3): 600–8. doi:10.1016/j.pain.2007.10.021. PMID 18054437. S2CID 2757963.
- ↑ Daly AE, Bialocerkowski AE (April 2009). "Does evidence support physiotherapy management of adult Complex Regional Pain Syndrome Type One? A systematic review". European Journal of Pain. 13 (4): 339–53. doi:10.1016/j.ejpain.2008.05.003. PMID 18619873. S2CID 207607466.
- ↑ "Graded Motor Imagery". Graded Motor Imagery. Archived from the original on 2014-01-02. Retrieved 2013-12-23.
- ↑ Duong, Silvia; Bravo, Daniela; Todd, Keith J.; Finlayson, Roderick J.; Tran, De Q. (2018-06-01). "Treatment of complex regional pain syndrome: an updated systematic review and narrative synthesis". Canadian Journal of Anesthesia/Journal canadien d'anesthésie. 65 (6): 658–684. doi:10.1007/s12630-018-1091-5. ISSN 1496-8975. Archived from the original on 2021-08-28. Retrieved 2021-03-09.
- ↑ O'Connell, NE; Wand, BM; Gibson, W; Carr, DB; Birklein, F; Stanton, TR (28 July 2016). "Local anaesthetic sympathetic blockade for complex regional pain syndrome". The Cochrane Database of Systematic Reviews (Submitted manuscript). 7: CD004598. doi:10.1002/14651858.CD004598.pub4. PMC 7202132. PMID 27467116. Archived from the original on 21 July 2018. Retrieved 7 November 2018.
- ↑ Kharkar S, Ambady P, Venkatesh Y, Schwartzman RJ (2011). "Intramuscular botulinum toxin in complex regional pain syndrome: case series and literature review". Pain Physician. 14 (5): 419–24. PMID 21927045. Archived from the original on 2015-02-03.
- ↑ Azari, P; Lindsay, DR; Briones, D; Clarke, C; Buchheit, T; Pyati, S (1 March 2012). "Efficacy and safety of ketamine in patients with complex regional pain syndrome: a systematic review". CNS Drugs. 26 (3): 215–28. doi:10.2165/11595200-000000000-00000. PMID 22136149. S2CID 26780542.
- ↑ Schwartzman, RJ; Alexander, GM; Grothusen, JR (May 2011). "The use of ketamine in complex regional pain syndrome: possible mechanisms". Expert Review of Neurotherapeutics. 11 (5): 719–34. doi:10.1586/ern.11.31. PMID 21539489. S2CID 18063794.
- ↑ 53.0 53.1 Niesters, M; Martini, C; Dahan, A (February 2014). "Ketamine for chronic pain: risks and benefits". British Journal of Clinical Pharmacology. 77 (2): 357–67. doi:10.1111/bcp.12094. PMC 4014022. PMID 23432384.
- ↑ Brunner F, Schmid A, Kissling R, Held U, Bachmann LM (January 2009). "Biphosphonates for the therapy of complex regional pain syndrome I--systematic review" (PDF). European Journal of Pain (Submitted manuscript). 13 (1): 17–21. doi:10.1016/j.ejpain.2008.03.005. PMID 18440845. S2CID 11615640. Archived (PDF) from the original on 2018-07-19. Retrieved 2018-11-07.
- ↑ "Complex Regional Pain Syndrome Fact Sheet | National Institute of Neurological Disorders and Stroke". Archived from the original on 2021-03-01. Retrieved 2019-01-07.
- ↑ Stengel, M; Binder, A; Baron, R (2007). "Update on the diagnosis and management of complex regional pain syndrome". Adv Pain Manage. 3 (1): 96–104.
- ↑ Taylor RS, Van Buyten JP, Buchser E (February 2006). "Spinal cord stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors". European Journal of Pain. 10 (2): 91–101. doi:10.1016/j.ejpain.2005.02.004. PMID 16310712. S2CID 27988384.
- ↑ Visnjevac, O; Costandi, S; Patel, BA; Azer, G; Agarwal, P; Bolash, R; Mekhail, NA (April 2017). "A Comprehensive Outcome-Specific Review of the Use of Spinal Cord Stimulation for Complex Regional Pain Syndrome". Pain Practice. 17 (4): 533–545. doi:10.1111/papr.12513. PMID 27739179. S2CID 20443171.
- ↑ vauthors=Deer T, et al | Dorsal root ganglion stimulation yielded higher treatment success for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial | Pain |volume=158 | issue=4| pages=669-681 | pmid=5359787 | doi=10.197/j.pain.0000000000000814
- ↑ Stanton-Hicks M, Baron R, Boas R, et al. (June 1998). "Complex Regional Pain Syndromes: guidelines for therapy". The Clinical Journal of Pain. 14 (2): 155–66. doi:10.1097/00002508-199806000-00012. PMID 9647459.
- ↑ Bodde MI, Dijkstra PU, den Dunnen WF, Geertzen JH (October 2011). "Therapy-resistant complex regional pain syndrome type I: to amputate or not?". The Journal of Bone and Joint Surgery. American Volume. 93 (19): 1799–805. doi:10.2106/JBJS.J.01329. hdl:11370/64f97da0-c435-4382-bd23-e0d91dad0c7e. PMID 22005865. Archived from the original on 2021-02-08. Retrieved 2019-07-07.
- ↑ "Complex Regional Pain Syndrome (CRPS): Management and Treatment". Cleveland Clinic. Archived from the original on 7 August 2020. Retrieved 10 August 2020.
- ↑ "RSDSA :: Reflex Sympathetic Dystrophy Syndrome Association". Rsds.org. 2010-01-21. Archived from the original on 2010-03-24. Retrieved 2010-04-10.
- ↑ Abu-Arafeh H, Abu-Arafeh I (February 2016). "Complex regional pain syndrome in children: incidence and clinical characteristics". Arch Dis Child. 101 (8): archdischild–2015–310233. doi:10.1136/archdischild-2015-310233. PMID 27005945. S2CID 35072465.
- ↑ Mitchell, S.W. (1872). Injuries of Nerves and their Consequences. Philadelphia: JB Lippincott. 377 pages.
- ↑ Richards RL (January 1967). "The term 'causalgia'". Medical History. 11 (1): 97–9. doi:10.1017/s0025727300011789. PMC 1033672. PMID 5341040.
- ↑ EVANS JA (March 1947). "Reflex sympathetic dystrophy; report on 57 cases". Annals of Internal Medicine. 26 (3): 417–26. doi:10.7326/0003-4819-26-3-417. PMID 20288177.
- ↑ Noordenbos, W. (1959). PAIN Problems pertaining to the transmission of nerve impulses which give rise to pain. Amsterdam: Elsevier.[page needed]
- ↑ Stanton-Hicks M, Jänig W, Hassenbusch S, Haddox JD, Boas R, Wilson P (October 1995). "Reflex sympathetic dystrophy: changing concepts and taxonomy". Pain. 63 (1): 127–33. doi:10.1016/0304-3959(95)00110-E. PMID 8577483. S2CID 1085473.
- ↑ McCabe, C. S. (2003). "A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1)". Rheumatology (Oxford, England). Oxford University Press (OUP). 42 (1): 97–101. doi:10.1093/rheumatology/keg041. ISSN 1460-2172. PMID 12509620.
- ↑ TREND homepage Archived December 24, 2009, at the Wayback Machine.
- ↑ Nia Frazier https://m.facebook.com/DrHollyFrazier/posts/thank-you-all-for-tuning-into-tonights-episode-of-dance-moms-this-was-a-very-per/213060555505579/ Archived 2021-08-28 at the Wayback Machine
- ↑ Paula Abdul "Archived copy". Archived from the original on 2015-06-02. Retrieved 2015-06-02.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ LA Times: Jill Kinmont Boothe is still going strong http://articles.latimes.com/2011/may/22/sports/la-sp-crowe-20110523 Archived 2011-11-14 at the Wayback Machine
- ↑ Gemma Collis http://www.paralympics.org.uk/gb/athletes/gemma-collis[full citation needed][permanent dead link]
- ↑ Shin Dong-Wook http://www.allkpop.com/2013/01/actor-shin-dong-wook-suffering-from-rare-disease Archived 2013-01-14 at the Wayback Machine
- ↑ Forest Tennant, MD, DrPH (July–August 2007). "Howard Hughes & Pseudoaddiction" (PDF). Practical Pain Management. 6 (7): 12–29. Archived from the original (PDF) on September 25, 2007. Retrieved January 7, 2011.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Rachel Morris http://www.paralympics.org.uk/gb/athletes/rachel-morris Archived 2012-09-01 at the Wayback Machine
- ↑ Cynthia Toussaint http://www.lifescript.com/health/centers/pain/articles/how_a_hamstring_tear_derailed_a_dancers_life.aspx Archived 2015-06-27 at the Wayback Machine
- ↑ BBC https://www.bbc.co.uk/sport/disability-sport/27064013 Archived 2016-06-26 at the Wayback Machine
- ↑ "Radene Marie Cook". 2017-12-02. Archived from the original on 2018-07-10. Retrieved 2018-07-10.
- ↑ "De Standaard - Rolstoelatlete Marieke Vervoort is overleden". Archived from the original on 2019-10-22. Retrieved 2019-10-22.
- ↑ "Bruno Soriano, la lesión más larga". Archived from the original on 2020-12-03. Retrieved 2020-11-20.
- ↑ Bergadano A, Moens Y, Schatzmann U (May 2006). "Continuous extradural analgesia in a cow with complex regional pain syndrome". Veterinary Anaesthesia and Analgesia. 33 (3): 189–92. doi:10.1111/j.1467-2995.2005.00245.x. PMID 16634945.
External links
| Classification | |
|---|---|
| External resources |
- Pages with script errors
- Webarchive template wayback links
- All articles with incomplete citations
- Articles with incomplete citations from June 2014
- Articles with invalid date parameter in template
- Wikipedia articles needing page number citations from June 2014
- CS1 maint: archived copy as title
- All articles with dead external links
- Articles with dead external links from July 2020
- Articles with permanently dead external links
- CS1 maint: multiple names: authors list
- Articles with hatnote templates targeting a nonexistent page
- All articles with unsourced statements
- Articles with unsourced statements from October 2020
- Articles with unsourced statements from December 2020
- Articles with unsourced statements from December 2013
- Articles with Curlie links
- Nerve, nerve root and plexus disorders
- Syndromes of unknown causes
- Chronic pain syndromes
- Neurocutaneous conditions
- Osteopathies
- Pain
- RTT