Comparison of phytocannabinoids
Cannabinoids (/kəˈnæbənɔɪdzˌ ˈkænəbənɔɪdz/) are compounds found in the cannabis plant or synthetic compounds that can interact with the endocannabinoid system.[1][2] The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (Delta-9-THC), the primary intoxicating compound in cannabis.[3][4] Cannabidiol (CBD) is another major constituent of some cannabis plants.[5] Conversion of CBD to THC can occur when CBD is heated to temperatures between 250–300 °C, potentially leading to its partial transformation into THC.[6]
At least 113 distinct cannabinoids have been isolated from cannabis.[7] This article gives comparative structures of some of the more common natural and synthetic cannabinoids, as well as showing structures of legally banned and sanctioned cannabinoids.
Structures
| Cannabinoid | 2D Structure | 3D Structure |
|---|---|---|
| CBC | 
|

|
| CBCV | 
|

|
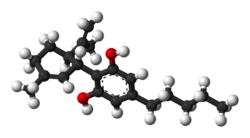
| CBD | 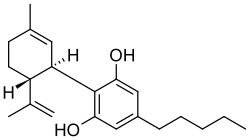
|
|
| CBDD | 
|

|
| CBDH | 
|

|
| CBDO | 
|
|
| CBDP | 
|

|
| CBDV | 
|

|
| CBE | 
|

|
| CBG | 
|

|
| CBGV | 
|

|
| CBL | 
|

|
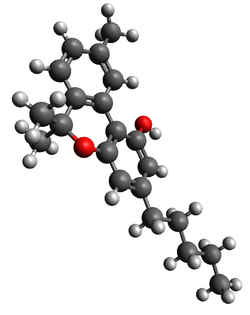
| CBN | 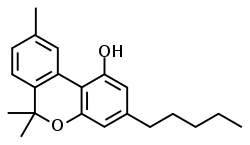
|
|
| CBND | 
|

|
| CBTC | 
|

|
| CBV | 
|

|
| delta-8-THC | 
|

|
| delta-10-THC | 
|

|
| THC | 
|
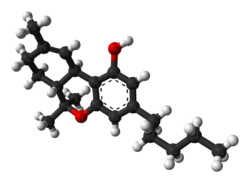
|
| THCC | 
|

|
| THCB | 
|

|
| THCH | 
|

|
| THCP | 
|

|
| THCV | 
|

|
Legality
| Cannabinoid | Quasi-psychedelic | Drug precursor | Legal status |
|---|---|---|---|
| CBCA, CBC | Legal in most countries | ||
| CBCVA, CBCV | |||
| CBDA, CBD | No | THC[6] | See Cannabidiol § Legal status |
| CBDD | |||
| CBDH | |||
| CBDO | |||
| CBDPA, CBDP | |||
| CBDVA, CBDV | |||
| CBEA, CBE | |||
| CBGA, CBG | |||
| CBGVA, CBGV | |||
| CBLA, CBL | |||
| CBNA, CBN | |||
| CBNDA, CBND | |||
| CBTA, CBT | |||
| CBVA, CBV | |||
| delta-8-THC | Yes | Legal in most countries | |
| delta-10-THC | Yes | Legal in most countries | |
| THCA, THC | Yes | UN Convention on Psychotropic Substances | |
| THCB | |||
| THCCA, THCC | |||
| THCH | Yes | Legal in most countries | |
| THCPA, THCP | Yes | Legal in most countries | |
| THCVA, THCV |
Thermal properties
Conversion temperatures
| Cyclization reaction | Temperature |
|---|---|
| CBD → THC | 250 °C (482 °F) to 300 °C (572 °F)[6] |
Decarboxylation temperatures
All cannabinoids listed here and their acids are found naturally in the plant to varying degrees.
| Decarboxylation reaction | Temperature |
|---|---|
| CBCA → CBC | |
| CBCVA → CBCV | |
| CBDA → CBD | |
| CBDPA → CBDP | |
| CBDVA → CBDV | |
| CBEA → CBE | |
| CBGA → CBG | |
| CBGVA → CBGV | |
| CBLA → CBL | |
| CBNA → CBN | |
| CBNDA → CBND | |
| CBTA → CBT | |
| CBVA → CBV | |
| THCA → THC | 230 °F (110 °C) to 250 °F (121 °C)[8][9] |
| THCCA → THCC | |
| THCPA → THCP | |
| THCVA → THCV |
This section needs additional citations for verification. (November 2021) |
Upon heating, cannabinoid acids decarboxylate to give their psychoactive cannabinoid. For example, Delta-9-tetrahydrocannabinol (THC) is the main psychoactive compound found in cannabis and is responsible for the "high" feeling when consumed. However, cannabis does not naturally contain significant amounts of THC. Instead, tetrahydrocannabinolic acid (THCA) is found naturally in raw and live cannabis and is non-intoxicating. Over time, THCA slowly converts to THC through a process of decarboxylation over the course of roughly a year, but can be sped up with exposure to high temperatures. When heated under conditions of 110 °C, decarboxylation generally occurs in 30–45 minutes. The decarboxylated THCA (THC) is added to cannabis edibles, as THCA is not orally active. When consumed orally, the liver breaks down and metabolizes THC into the more potent 11-hydroxy-THC.
Vaporization temperatures
This section needs additional citations for verification. (November 2021) |
Dry-herb vaporizers can be used to inhale cannabis in its flower form. There are 483 identifiable chemical constituents known to exist in the cannabis plant, and at least 85 different cannabinoids have been isolated from the plant.[10] The aromatic terpenoids begin to vaporize at 126.0 °C (258.8 °F), but the more bioactive tetrahydrocannabinol (THC), and other cannabinoids also found in cannabis (often legally sold as cannabinoid isolates) like cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN), do not vaporize until near their respective boiling points.
The cannabinoids listed here are found in the plant but only in trace amounts. However, they have also been extracted and sold as isolates online. Third party certification may help ensure buyers to avoid synthetic cannabinoids.
| Cannabinoid | Boiling point |
|---|---|
| CBC | 220 °C (428 °F)[11] |
| CBCV | |
| CBD | 160 °C (320 °F)-180 °C (356 °F)[11] |
| CBDD | ? |
| CBDO | |
| CBDP | |
| CBDV | |
| CBE | |
| CBG | 185 °C (365 °F)[12] |
| CBGV | |
| CBL | |
| CBN | 185 °C (365 °F)[11] |
| CBT | |
| CBV | |
| delta-8-THC | 175 °C (347 °F)-178 °C (352 °F)[11] |
| delta-10-THC | ? |
| THC | 157 °C (315 °F)[11] |
| THCB | |
| THCC | |
| THCP | |
| THCV | <220[11] |
Structural scheduling
| Cannabigerol-type (CBG) | ||||
|---|---|---|---|---|

Cannabigerol |

Cannabigerol |
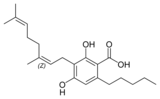
Cannabinerolic acid A |

Cannabigerovarin |
|
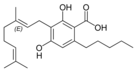
Cannabigerolic acid A |

Cannabigerolic acid A |

Cannabigerovarinic acid A |
||
| Cannabichromene-type (CBC) | ||||

(±)-Cannabichromene |

(±)-Cannabichromenic acid A |

(±)-Cannabivarichromene, (±)-Cannabichromevarin |

(±)-Cannabichromevarinic |
|
| Cannabidiol-type (CBD) | ||||
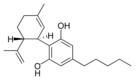
(−)-Cannabidiol |

Cannabidiol |

Cannabidiol-C4 |

(−)-Cannabidivarin |

Cannabidiorcol |
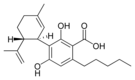
Cannabidiolic acid |

Cannabidivarinic acid |
|||
| Cannabinodiol-type (CBND) | ||||

Cannabinodiol |

Cannabinodivarin |
|||
| Tetrahydrocannabinol-type (THC) | ||||

Δ9-Tetrahydrocannabinol |

Δ9-Tetrahydrocannabinol-C4 |

Δ9-Tetrahydrocannabivarin |

Δ9-Tetrahydrocannabiorcol | |

Δ9-Tetrahydro- |

Δ9-Tetrahydro- |

Δ9-Tetrahydro- |
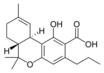
Δ9-Tetrahydro- |

Δ9-Tetrahydro- |

(−)-Δ8-trans-(6aR,10aR)- |

(−)-Δ8-trans-(6aR,10aR)- |

(−)-(6aS,10aR)-Δ9- |
||
| Cannabinol-type (CBN) | ||||
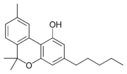
Cannabinol |

Cannabinol-C4 |

Cannabivarin |

Cannabinol-C2 |

Cannabiorcol |
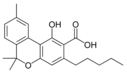
Cannabinolic acid A |

Cannabinol methyl ether |
|||
| Cannabitriol-type (CBT) | ||||

(−)-(9R,10R)-trans- |

(+)-(9S,10S)-Cannabitriol |

(±)-(9R,10S/9S,10R)- |

(−)-(9R,10R)-trans- |

(±)-(9R,10R/9S,10S)- |
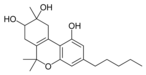
8,9-Dihydroxy-Δ6a(10a)- |

Cannabidiolic acid A |

(−)-(6aR,9S,10S,10aR)- |

(−)-6a,7,10a-Trihydroxy- |
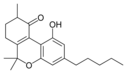
10-Oxo-Δ6a(10a)- |
| Cannabielsoin-type (CBE) | ||||

(5aS,6S,9R,9aR)- |
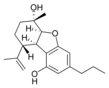
(5aS,6S,9R,9aR)- |
|||

(5aS,6S,9R,9aR)- |

(5aS,6S,9R,9aR)- |

(5aS,6S,9R,9aR)- |
||

Cannabiglendol-C3 |

Dehydrocannabifuran |

Cannabifuran |
||
| Isocannabinoids | ||||

(−)-Δ7-trans-(1R,3R,6R)- |

(±)-Δ7-1,2-cis- |

(−)-Δ7-trans-(1R,3R,6R)- |
||
| Cannabicyclol-type (CBL) | ||||
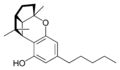
(±)-(1aS,3aR,8bR,8cR)- |

(±)-(1aS,3aR,8bR,8cR)- |

(±)-(1aS,3aR,8bR,8cR)- |
||
| Cannabicitran-type (CBT) | ||||

Cannabicitran |
||||
| Cannabichromanone-type (CBCN) | ||||

Cannabichromanone |

Cannabichromanone-C3 |

Cannabicoumaronone |
||
References
- ^ Abyadeh M, Gupta V, Paulo JA, Gupta V, Chitranshi N, Godinez A, et al. (September 2021). "A Proteomic View of Cellular and Molecular Effects of Cannabis". Biomolecules. 11 (10): 1411–1428. doi:10.3390/biom11101411. PMC 8533448. PMID 34680044.
- ^ "Marijuana, also called: Cannabis, Ganja, Grass, Hash, Pot, Weed". Medline Plus. 3 July 2017.
- ^ Lambert DM, Fowler CJ (August 2005). "The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications". Journal of Medicinal Chemistry. 48 (16): 5059–5087. doi:10.1021/jm058183t. PMID 16078824.
- ^ Pertwee R, ed. (2005). Cannabinoids. Springer-Verlag. p. 2. ISBN 978-3-540-22565-2.
- ^ "Bulletin on Narcotics – 1962 Issue 3 – 004". UNODC (United Nations Office of Drugs and Crime). 1962-01-01. Retrieved 2014-01-15.
- ^ a b c Czégény Z, Nagy G, Babinszki B, Bajtel Á, Sebestyén Z, Kiss T, et al. (April 2021). "CBD, a precursor of THC in e-cigarettes". Scientific Reports. 11 (1): 8951. Bibcode:2021NatSR..11.8951C. doi:10.1038/s41598-021-88389-z. PMC 8076212. PMID 33903673.
- ^ Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. (February 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–331. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472.
- ^ Wang M, Wang YH, Avula B, Radwan MM, Wanas AS, van Antwerp J, et al. (1 December 2016). "Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry". Cannabis and Cannabinoid Research. 1 (1): 262–271. doi:10.1089/can.2016.0020. PMC 5549281. PMID 28861498.
- ^ Wang M, Wang YH, Avula B, Radwan MM, Wanas AS, van Antwerp J, et al. (2016). "Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry". Cannabis and Cannabinoid Research. 1 (1): 262–271. doi:10.1089/can.2016.0020. PMC 5549281. PMID 28861498.
- ^ El-Alfy AT, Ivey K, Robinson K, Ahmed S, Radwan M, Slade D, et al. (June 2010). "Antidepressant-like effect of delta9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L". Pharmacology, Biochemistry, and Behavior. 95 (4): 434–442. doi:10.1016/j.pbb.2010.03.004. PMC 2866040. PMID 20332000.
- ^ a b c d e f "Phytocannabinoid Boiling Points" (PDF). projectcbd.org. Archived (PDF) from the original on 2019-04-08. Retrieved 20 August 2021.
- ^ "CompTox Chemicals Dashboard". comptox.epa.gov. Retrieved 10 April 2024.
- ^ "Table of Natural Cannabinoids" (PDF).
