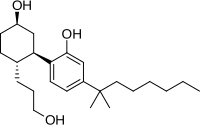
CP 55,940
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H40O3 |
| Molar mass | 376.581 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
CP 55,940 is a synthetic cannabinoid which mimics the effects of naturally occurring THC (one of the psychoactive compounds found in cannabis). CP 55,940 was created by Pfizer in 1974 but was never marketed. It is currently used as a research tool to study the endocannabinoid system.[1]
Pharmacology
CP 55,940 is 45 times more potent than Δ9-THC, and fully antagonized by rimonabant (SR141716A).[2] It is considered a full agonist at both CB1 and CB2 receptors and has Ki values of 0.58 nM and 0.68 nM respectively, but is an antagonist at GPR55, the putative "CB3" receptor.[3] CP 55,940 binding has been detected in the cytosol of rat brain cerebral cortex.[4] It can upregulate 5-HT2A receptors in mice.[5]
In vitro studies
CP 55,940 induced cell death in NG 108-15 Mouse neuroblastoma x Rat glioma hybrid brain cancer (genetically engineered mouse x rat brain cancer) cells.[6][7]
In vivo studies
CP 55,940 showed protective effects on rat brain mitochondria upon paraquat exposure.[8]
It also showed neuroprotective effects by reducing intracellular calcium release and reducing hippocampal cell death in cultured neurons subjected to high levels of NMDA.[9]
See also
References
- ^ Glass M, Dragunow M, Faull RL (March 1997). "Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain". Neuroscience. 77 (2): 299–318. doi:10.1016/s0306-4522(96)00428-9. PMID 9472392. S2CID 40213209.
- ^ Rinaldi-Carmona M, Pialot F, Congy C, Redon E, Barth F, Bachy A, et al. (1996). "Characterization and distribution of binding sites for [3H]-SR 141716A, a selective brain (CB1) cannabinoid receptor antagonist, in rodent brain". Life Sciences. 58 (15): 1239–47. doi:10.1016/0024-3205(96)00085-9. PMID 8614277.
- ^ Kapur A, Zhao P, Sharir H, Bai Y, Caron MG, Barak LS, Abood ME (October 2009). "Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands". The Journal of Biological Chemistry. 284 (43): 29817–27. doi:10.1074/jbc.M109.050187. PMC 2785612. PMID 19723626.
- ^ Qureshi J, Saady M, Cardounel A, Kalimi M (April 1998). "Identification and characterization of a novel synthetic cannabinoid CP 55,940 binder in rat brain cytosol". Molecular and Cellular Biochemistry. 181 (1–2): 21–27. doi:10.1023/A:1006855504094. PMID 9562238. S2CID 6200670.
- ^ Franklin JM, Carrasco GA (March 2013). "Cannabinoid receptor agonists upregulate and enhance serotonin 2A (5-HT(2A)) receptor activity via ERK1/2 signaling". Synapse. 67 (3): 145–59. doi:10.1002/syn.21626. PMC 3552103. PMID 23151877.
- ^ Tomiyama K, Funada M (November 2011). "Cytotoxicity of synthetic cannabinoids found in "Spice" products: the role of cannabinoid receptors and the caspase cascade in the NG 108-15 cell line". Toxicology Letters. 207 (1): 12–7. doi:10.1016/j.toxlet.2011.08.021. PMID 21907772.
- ^ "General Cell Collection: NG108-15". Public Health England Culture Collections.
- ^ Velez-Pardo C, Jimenez-Del-Rio M, Lores-Arnaiz S, Bustamante J (September 2010). "Protective effects of the synthetic cannabinoids CP55,940 and JWH-015 on rat brain mitochondria upon paraquat exposure". Neurochemical Research. 35 (9): 1323–32. doi:10.1007/s11064-010-0188-1. hdl:11336/67604. PMID 20514518. S2CID 821457.
- ^ Zhuang SY, Bridges D, Grigorenko E, McCloud S, Boon A, Hampson RE, Deadwyler SA (June 2005). "Cannabinoids produce neuroprotection by reducing intracellular calcium release from ryanodine-sensitive stores". Neuropharmacology. 48 (8): 1086–96. doi:10.1016/j.neuropharm.2005.01.005. PMID 15910885. S2CID 14953725.
- Articles with short description
- Short description matches Wikidata
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Primary alcohols
- Cyclohexanols
- Cannabinoids
- Designer drugs
- Drugs developed by Pfizer
- Phenols
- CB1 receptor agonists
- CB2 receptor agonists
