Asenapine
 | |
 | |
| Names | |
|---|---|
| Trade names | Saphris, Sycrest, Secuado, others |
| Other names | Asenapine maleate, ORG-5222 |
| |
| Clinical data | |
| Drug class | Atypical antipsychotic |
| Main uses | Schizophrenia, mania in bipolar disorder[1][2] |
| Side effects | Sleepiness, dizziness, weight gain, movement disorders, numbness within the mouth[1] |
| Pregnancy category |
|
| Routes of use | Under the tongue |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610015 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 35% (sublingual), <2% (by mouth)[4][5][3][6] |
| Protein binding | 95%[4][5][3][6] |
| Metabolism | hepatic (glucurinodation by UGT1A4 and oxidative metabolism by CYP1A2)[4][5][3][6] |
| Elimination half-life | 24 hours[4][5][3][6] |
| Excretion | Kidney (50%), Faecal (40%; ~5–16% as unchanged drug in faeces)[4][5][3][6] |
| Chemical and physical data | |
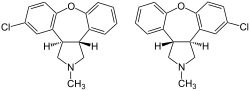
| Formula | C17H16ClNO |
| Molar mass | 285.77 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Asenapine, sold under the brand name Saphris among others, is an atypical antipsychotic used to treat schizophrenia and mania in bipolar disorder.[1][2] Use in older people with dementia related psychosis may increase the risk of death.[1] It is used under the tongue.[7]
Common side effects include sleepiness, dizziness, weight gain, movement disorders, and numbness within the mouth.[1] Other side effects may include stroke, neuroleptic malignant syndrome, tardive dyskinesia, diabetes, low white blood cells, seizures, and QT prolongation.[1] How it works is not clear, but is believed to involve blocking serotonin and dopamine receptors.[2]
Asenapine was approved for medical use in the United States in 2009 and Europe in 2010.[1][2] The first generic versions were approved in 2020.[8] In the United Kingdom it costs the NHS about £100 per month as of 2021.[9] This amount in the United States is about 200 USD.[10]
Medical uses
Asenapine has been approved by the FDA for the acute treatment of adults with schizophrenia and acute treatment of manic or mixed episodes associated with bipolar I disorder with or without psychotic features in adults.[11] In Australia asenapine's approved (and also listed on the PBS) indications include the following:[12]
- Schizophrenia
- Treatment, for up to 6 months, of an episode of acute mania or mixed episodes associated with bipolar I disorder
- Maintenance treatment, as monotherapy, of bipolar I disorder
In the European Union and the United Kingdom, asenapine is only licensed for use as a treatment for acute mania in bipolar I disorder.[3][6][2]
Absorbed readily if administered sublingually, asenapine is poorly absorbed when swallowed.[13]
Schizophrenia
A Cochrane review found that while asenapine has some preliminary evidence that it improves positive, negative, and depressive symptoms, it does not have enough research to merit a certain recommendation of Asenapine for the treatment of schizophrenia.[14]
Mania
As for its efficacy in the treatment of acute mania, a recent meta-analysis showed that it produces comparatively small improvements in manic symptoms in patients with acute mania and mixed episodes than most other antipsychotic drugs (with the exception of ziprasidone) such as risperidone and olanzapine. Drop-out rates (in clinical trials) were also unusually high with asenapine.[15] According to a post-hoc analysis of two 3-week clinical trials it may possess some antidepressant effects in patients with acute mania or mixed episodes.[16]
Dosage
It is generally taken at 5 mg twice per day though may be increased up to 10 mg twice per day.[9]
Side effects
Side effect incidence[4][5][3][6]
Very common (>10%):
Common (1-10%):
- Weight gain
- Increased appetite
- Extrapyramidal side effects (EPS; such as dystonia, akathisia, dyskinesia, muscle rigidity, parkinsonism)
- Sedation
- Dizziness
- Dysgeusia
- Oral hypoaesthesia
- Increased alanine aminotransferase
- Fatigue
Uncommon (0.1-1%):
- Hyperglycaemia — elevated blood glucose (sugar)
- Syncope
- Seizure
- Dysarthria
- sinus bradycardia
- Bundle branch block
- QTc interval prolongation (has a relatively low risk for causing QTc interval prolongation.[17][18])
- sinus tachycardia
- Orthostatic hypotension
- Hypotension
- Swollen tongue
- Dysphagia (difficulty swallowing)
- Glossodynia
- Oral paraesthesia
Rare (0.01-0.1%):
- Neuroleptic malignant syndrome (Combination of fever, muscle stiffness, faster breathing, sweating, reduced consciousness, and sudden change in blood pressure and heart rate)
- Tardive dyskinesia
- Speech disturbance
- Rhabdomyolysis
- Angioedema
- Blood dyscrasias such as agranulocytosis, leukopenia and neutropenia
- Accommodation disorder[clarification needed]
- Pulmonary embolism
- Gynaecomastia
- Galactorrhoea
Unknown incidence
- Allergic reaction
- Restless legs syndrome
- Nausea
- Oral mucosal lesions (ulcerations, blistering and inflammation)
- Salivary hypersecretion
- Hyperprolactinaemia
Asenapine seems to have a relatively low weight gain liability for an atypical antipsychotic (which are notorious for their metabolic side effects) and a 2013 meta-analysis found significantly less weight gain (SMD [standard mean difference in weight gained in those on placebo vs. active drug]: 0.23; 95% CI: 0.07-0.39) than, paliperidone (SMD: 0.38; 95% CI: 0.27-0.48), risperidone (SMD: 0.42; 95% CI: 0.33-0.50), quetiapine (SMD: 0.43; 95% CI: 0.34-0.53), sertindole (SMD: 0.53; 95% CI: 0.38-0.68), chlorpromazine (SMD: 0.55; 95% CI: 0.34-0.76), iloperidone (SMD: 0.62; 95% CI: 0.49-0.74), clozapine (SMD: 0.65; 95% CI: 0.31-0.99), zotepine (SMD: 0.71; 95% CI: 0.47-0.96) and olanzapine (SMD: 0.74; 95% CI: 0.67-0.81) and approximately (that is, no statistically significant difference at the p=0.05 level) as much as weight gain as aripiprazole (SMD: 0.17; 95% CI: 0.05-0.28), lurasidone (SMD: 0.10; 95% CI: –0.02-0.21), amisulpride (SMD: 0.20; 95% CI: 0.05-0.35), haloperidol (SMD: 0.09; 95% CI: 0.00-0.17) and ziprasidone (SMD: 0.10; 95% CI: –0.02-0.22).[19] Its potential for elevating plasma prolactin levels seems relatively limited too according to this meta-analysis.[19] This meta-analysis also found that asenapine has approximately the same odds ratio (3.28; 95% CI: 1.37-6.69) for causing sedation [compared to placebo-treated patients] as olanzapine (3.34; 95% CI: 2.46-4.50]) and haloperidol (2.76; 95% CI: 2.04-3.66) and a higher odds ratio (although not significantly) for sedation than aripiprazole (1.84; 95% CI: 1.05-3.05), paliperidone (1.40; 95% CI: 0.85-2.19) and amisulpride (1.42; 95% CI: 0.72 to 2.51) to name a few and is hence a mild-moderately sedating antipsychotic.[19] The same meta-analysis suggested that asenapine had a relatively high risk of extrapyramidal symptoms compared to other atypical antipsychotics but a lower risk than first-generation or typical antipsychotics.[19]
Discontinuation
For all antipsychotics, the British National Formulary recommends a gradual dose reduction when discontinuing to avoid acute withdrawal syndrome or rapid relapse.[20] Symptoms of withdrawal commonly include nausea, vomiting, and loss of appetite.[21] Other symptoms may include restlessness, increased sweating, and trouble sleeping.[21] Less commonly there may be a feeling of the world spinning, numbness, or muscle pains.[21] Symptoms generally resolve after a short period of time.[21]
There is tentative evidence that discontinuation of antipsychotics can result in psychosis.[22] It may also result in recurrence of the condition that is being treated.[23] Rarely tardive dyskinesia can occur when the medication is stopped.[21]
Pharmacology
It was chemically derived via altering the chemical structure of the tetracyclic (atypical) antidepressant, mianserin.[24]
Pharmacodynamics
| Site | pKi | Ki (nM) | Action |
|---|---|---|---|
| 5-HT1A | 8.6 | 2.5 | Partial agonist |
| 5-HT1B | 8.4 | 4.0 | Antagonist |
| 5-HT2A | 10.2 | 0.06 | Antagonist |
| 5-HT2B | 9.8 | 0.16 | Antagonist |
| 5-HT2C | 10.5 | 0.03 | Antagonist |
| 5-HT5A | 8.8 | 1.6 | Antagonist |
| 5-HT6 | 9.5 | 0.25 | Antagonist |
| 5-HT7 | 9.9 | 0.13 | Antagonist |
| α1 | 8.9 | 1.2 | Antagonist |
| α2A | 8.9 | 1.2 | Antagonist |
| α2B | 9.5 | 0.32 | Antagonist |
| α2C | 8.9 | 1.2 | Antagonist |
| D1 | 8.9 | 1.4 | Antagonist |
| D2 | 8.9 | 1.3 | Antagonist |
| D3 | 9.4 | 0.42 | Antagonist |
| D4 | 9.0 | 1.1 | Antagonist |
| H1 | 9.0 | 1.0 | Antagonist |
| H2 | 8.2 | 6.2 | Antagonist |
| mACh | <5 | 8128 | Antagonist |
Asenapine shows high affinity (pKi) for numerous receptors, including the serotonin 5-HT1A (8.6), 5-HT1B (8.4), 5-HT2A (10.2), 5-HT2B (9.8), 5-HT2C (10.5), 5-HT5A (8.8), 5-HT6 (9.5), and 5-HT7 (9.9) receptors, the adrenergic α1 (8.9), α2A (8.9), α2B (9.5), and α2C (8.9) receptors, the dopamine D1 (8.9), D2 (8.9), D3 (9.4), and D4 (9.0) receptors, and the histamine H1 (9.0) and H2 (8.2) receptors. It has much lower affinity (pKi < 5) for the muscarinic acetylcholine receptors. Asenapine behaves as a partial agonist at the 5-HT1A receptors.[26] At all other targets asenapine is an antagonist.[25] As of November 2010 asenapine is also in clinical trials at UC Irvine to treat stuttering.
Even relative to other atypical antipsychotics, asenapine has unusually high affinity for the 5-HT2A, 5-HT2C, 5-HT6, and 5-HT7 receptors, and very high affinity for the α2 and H1 receptors.[25]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "DailyMed - SAPHRIS- asenapine maleate tablet". dailymed.nlm.nih.gov. Archived from the original on 13 November 2021. Retrieved 16 January 2022.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Sycrest EPAR". European Medicines Agency (EMA). Archived from the original on 15 August 2020. Retrieved 9 September 2020.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "Sycrest 5mg sublingual tablets - Summary of Product Characteristics (SmPC)". (emc). Archived from the original on 23 September 2020. Retrieved 9 September 2020.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 "Product Information Saphris (asenapine maleate)" (PDF). TGA eBusiness Services. Merck Sharp & Dohme (Australia) Pty Limited. 14 January 2013. Archived from the original on 2 April 2019. Retrieved 23 October 2013.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "Saphris (asenapine maleate) tablet". DailyMed. Organon Pharmaceuticals. March 2013. Archived from the original on 3 December 2013. Retrieved 23 October 2013.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 "Product information Sycrest – EMEA/H/C/001177 –II/0012" (PDF). European Medicines Agency. N.V. Organon. 21 February 2013. Archived (PDF) from the original on 28 July 2017. Retrieved 23 October 2013.
- ↑ "Asenapine". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 16 January 2022. Retrieved 16 January 2022.
- ↑ Research, Center for Drug Evaluation and (23 February 2021). "2020 First Generic Drug Approvals". FDA. Archived from the original on 26 September 2021. Retrieved 16 January 2022.
- ↑ 9.0 9.1 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 376. ISBN 978-0857114105.
- ↑ "Asenapine Prices, Coupons & Savings Tips". GoodRx. Archived from the original on 23 October 2016. Retrieved 16 January 2022.
- ↑ 11.0 11.1 "Saphris (asenapine) prescribing information" (PDF). Schering Corporation. 2009-08-01. Archived from the original (PDF) on 2009-11-22. Retrieved 2009-09-05.
- ↑ Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ↑ Stoner, Steven (2012). "Asenapine: a clinical review of a second-generation antipsychotic". Clinical Therapeutics. 34 (5): 1023–40. doi:10.1016/j.clinthera.2012.03.002. PMID 22494521.
- ↑ Hay, A; Byers, A; Sereno, M (2015). "Asenapine versus placebo for schizophrenia". Cochrane Database of Systematic Reviews (11): CD011458.pub2. doi:10.1002/14651858.CD011458.pub2. PMC 6464872. PMID 26599405. Archived from the original on 2019-06-17. Retrieved 2021-11-01.
- ↑ Cipriani, A; Barbui, C; Salanti, G; Rendell, J; Brown, R; Stockton, S; Purgato, M; Spineli, LM; Goodwin, GM; Geddes, JR (October 2011). "Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis". Lancet. 378 (9799): 1306–1315. doi:10.1016/S0140-6736(11)60873-8. PMID 21851976. S2CID 25512763.
- ↑ Szegedi, A; Zhao, J; van Willigenburg, A; Nations, KR; Mackle, M; Panagides, J (June 2011). "Effects of asenapine on depressive symptoms in patients with bipolar I disorder experiencing acute manic or mixed episodes: a post hoc analysis of two 3-week clinical trials". BMC Psychiatry. 11: 101. doi:10.1186/1471-244X-11-101. PMC 3152513. PMID 21689438.
- ↑ Washington, Nicole B. (October 2012). "Which psychotropics carry the greatest risk of QTc prolongation?". Current Psychiatry. 11 (10): 36–39. Archived from the original on 15 April 2017. Retrieved 14 April 2017.
- ↑ Taylor, D; Paton, C; Shitij, K (2012). The Maudsley prescribing guidelines in psychiatry. West Sussex: Wiley-Blackwell. ISBN 978-0-470-97948-8.
- ↑ 19.0 19.1 19.2 19.3 Leucht, S; Cipriani, A; Spineli, L; Mavridis, D; Orey, D; Richter, F; Samara, M; Barbui, C; Engel, RR; Geddes, JR; Kissling, W; Stapf, MP; Lässig, B; Salanti, G; Davis, JM (September 2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis". Lancet. 382 (9896): 951–962. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019. S2CID 32085212.
- ↑ Joint Formulary Committee, BMJ, ed. (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192. ISBN 978-0-85369-845-6.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse.
- ↑ 21.0 21.1 21.2 21.3 21.4 Haddad, Peter; Haddad, Peter M.; Dursun, Serdar; Deakin, Bill (2004). Adverse Syndromes and Psychiatric Drugs: A Clinical Guide. OUP Oxford. p. 207–216. ISBN 9780198527480. Archived from the original on 2021-04-14. Retrieved 2021-11-01.
- ↑ Moncrieff J (July 2006). "Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse". Acta Psychiatrica Scandinavica. 114 (1): 3–13. doi:10.1111/j.1600-0447.2006.00787.x. PMID 16774655. S2CID 6267180.
- ↑ Sacchetti, Emilio; Vita, Antonio; Siracusano, Alberto; Fleischhacker, Wolfgang (2013). Adherence to Antipsychotics in Schizophrenia. Springer Science & Business Media. p. 85. ISBN 9788847026797. Archived from the original on 2021-04-14. Retrieved 2021-11-01.
- ↑ Minassian, A; Young, JW (August 2010). "Evaluation of the clinical efficacy of asenapine in schizophrenia". Expert Opinion on Pharmacotherapy. 11 (12): 2107–2115. doi:10.1517/14656566.2010.506188. PMC 2924192. PMID 20642375.
- ↑ 25.0 25.1 25.2 Shahid M, Walker GB, Zorn SH, Wong EH (2009). "Asenapine: a novel psychopharmacologic agent with a unique human receptor signature". J. Psychopharmacol. 23 (1): 65–73. doi:10.1177/0269881107082944. PMID 18308814. S2CID 206489515.
- ↑ Ghanbari, Ramez; El Mansari, Mostafa; Shahid, Mohammed; Blier, Pierre (March 2009). "Electrophysiological characterization of the effects of asenapine at 5-HT1A, 5-HT2A, α2-adrenergic and D2 receptors in the rat brain". European Neuropsychopharmacology. 19 (3): 177–187. doi:10.1016/j.euroneuro.2008.11.001. PMID 19116183. S2CID 140204044.
External links
| External sites: | |
|---|---|
| Identifiers: |
- "Asenapine maleate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-10-17. Retrieved 2021-11-01.
- Pages using duplicate arguments in template calls
- Drugs with non-standard legal status
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Multiple chemicals in Infobox drug
- Chemicals using indexlabels
- Drugs missing an ATC code
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Wikipedia articles needing clarification from February 2016
- Articles with invalid date parameter in template
- Articles with changed CASNo identifier
- Articles with changed EBI identifier
- 5-HT1A agonists
- AbbVie brands
- Alpha-2 blockers
- Atypical antipsychotics
- Chloroarenes
- Heterocyclic compounds with 4 rings
- Merck & Co. brands
- Nitrogen heterocycles
- Oxepines
- Oxygen heterocycles
- Schering-Plough brands
- Tertiary amines
- RTT
