Tetrandrine
This article needs more reliable medical references for verification or relies too heavily on primary sources. (March 2015) |  |

| |
| Names | |
|---|---|
| IUPAC name
9,20,21,25-tetramethoxy-15,30-dimethyl-7,23-dioxa-15,30-diazaheptacyclo[22.6.2.23,6.18,12.114,18.027,31.022,33]hexatriaconta-3,5,8(34),9,11,18,20,22(33),24(32),25,27(31),35-dodecaene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ECHA InfoCard | 100.208.615 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C38H42N2O6 | |
| Molar mass | 622.74988 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetrandrine, a bis-benzylisoquinoline alkaloid, is a calcium channel blocker.[1] It is isolated from the plant Stephania tetrandra,[2] and other Chinese and Japanese herbs.
Pharmacology
[edit]It has anti-inflammatory, immunologic and antiallergenic effects. It inhibits the degranulation of mast cells. It has a quinidine-like anti-arrhythmic effect. It has vasodilatory properties and can therefore reduce blood pressure.[3] Tetrandrine may have potential use for the treatment of liver disease[4] and liver cancer.[5][6][7] Tetrandrine has potential therapeutic value to prevent excess scarring/fibrosis in conjunctiva following trabeculectomy or in patients with severe conjunctival inflammation.[8] Tetrandrine has anti-inflammatory and anti-fibrogenic actions, which make tetrandrine and related compounds potentially useful in the treatment of lung silicosis, liver cirrhosis, and rheumatoid arthritis.[9] Tetrandrine has also been shown to inhibit entry of Ebola virus into host cells in vitro and showed therapeutic efficacy against Ebola in preliminary studies on mice.[10][1] Tetrandrine has also been studied and patented as a possible treatment for tinnitus.[11][12][13]
Biosynthesis
[edit]Tetrandrine is biosynthesized from a free radical coupled dimerization of S-N-methylcoclaurine:[14]
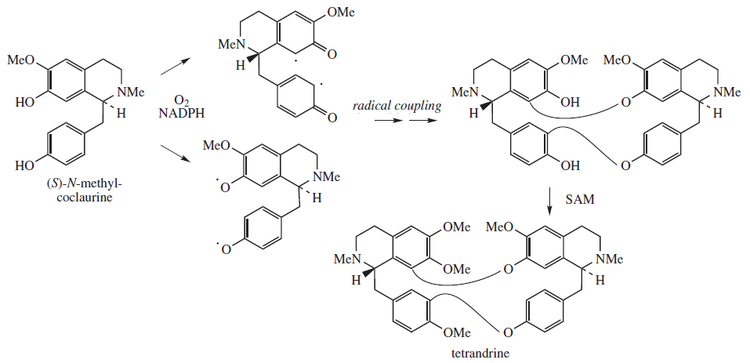
Synonyms
[edit]Synonyms include fanchinine, hanfangchin A, NSC 77037, (S,S)-(+)-tetrandrine, sinomenine A, TTD, tetrandrin, d-tetrandrine, and GW-201.[15][13]
References
[edit]- ^ a b Bhagya, N; Chandrashekar, KR (1 May 2016). "Tetrandrine – A molecule of wide bioactivity". Phytochemistry. 125: 5–13. Bibcode:2016PChem.125....5B. doi:10.1016/j.phytochem.2016.02.005. ISSN 0031-9422. PMID 26899361. Retrieved 24 February 2022.
- ^ Zhang, Lijin; Geng, Yanling; Duan, Wenjuan; Wang, Daijie; Fu, Maorun; Wang, Xiao (2009). "Ionic liquid-based ultrasound-assisted extraction of fangchinoline and tetrandrine from Stephaniae tetrandrae". Journal of Separation Science. 32 (20): 3550–3554. doi:10.1002/jssc.200900413. PMID 19764054.
- ^ Kwan, C. Y.; Achike, F. I. (2002). "Tetrandrine and related bis-benzylisoquinoline alkaloids from medicinal herbs: Cardiovascular effects and mechanisms of action". Acta Pharmacologica Sinica. 23 (12): 1057–68. PMID 12466042.
- ^ Feng, Dechun; Mei, Yunhua; Wang, Ying; Zhang, Bianhong; Wang, Chen; Xu, Lingyun (2008). "Tetrandrine protects mice from concanavalin A-induced hepatitis through inhibiting NF-κB activation". Immunology Letters. 121 (2): 127–133. doi:10.1016/j.imlet.2008.10.001. PMID 18992279.
- ^ Liu, Chaoyang; Gong, Ke; Mao, Xin; Li, Wenhua (2011). "Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma". International Journal of Cancer. 129 (6): 1519–1531. doi:10.1002/ijc.25817. PMID 21128229. S2CID 3163935.
- ^ Cheng, Zhixiang; Wang, Keming; Wei, Jia; Lu, Xiang; Liu, Baorui (2010). "Proteomic analysis of anti-tumor effects by tetrandrine treatment in HepG2 cells". Phytomedicine. 17 (13): 1000–1005. doi:10.1016/j.phymed.2010.03.018. PMID 20554191.
- ^ Deng, W. Y.; Luo, S. X.; Zhou, M. Q.; Li, N.; Chen, X. B.; Han, L. L. (2008). "The study of anti-tumor effect of Tetrandrine combined with Nedaplatin on human liver cancer cell line 7402". Zhong Yao Cai = Zhongyaocai = Journal of Chinese Medicinal Materials. 31 (10): 1522–5. PMID 19230406.
- ^ Kitano, Ai; Yamanaka, Osamu; Ikeda, Kazuo; Ishida-Nishikawa, Iku; Okada, Yuka; Shirai, Kumi; Saika, Shizuya (2008). "Tetrandrine Suppresses Activation of Human Subconjunctival Fibroblasts In Vitro". Current Eye Research. 33 (7): 559–565. doi:10.1080/02713680802220817. PMID 18600488. S2CID 37248080.
- ^ Kwan, C. Y.; Achike, F. I. (2002). "Tetrandrine and related bis-benzylisoquinoline alkaloids from medicinal herbs: Cardiovascular effects and mechanisms of action". Acta Pharmacologica Sinica. 23 (12): 1057–68. PMID 12466042.
- ^ Sakurai, Yasuteru; Kolokoltsov, Andrey A.; Chen, Cheng-Chang; Tidwell, Michael W.; Bauta, William E.; Klugbauer, Norbert; Grimm, Christian; Wahl-Schott, Christian; Biel, Martin; Davey, Robert A. (2015). "Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment". Science. 347 (6225): 995–998. Bibcode:2015Sci...347..995S. doi:10.1126/science.1258758. PMC 4550587. PMID 25722412.
- ^ US patent 10434097, Jianxin Bao & Xiaojie Chen, "Methods and compositions for treating hearing disorders", published 8 October 2019, assigned to Gateway Biotechnology Inc
- ^ "Targeting multiple signaling pathways for tinnitus prevention and treatment (R44DC018759)". HHS Tracking Accountability in Government Grants System (TAGGS). US Department of Health & Human Services. Archived from the original on 24 February 2022. Retrieved 24 February 2022.
- ^ a b Dyhrfjeld-Johnsen, Jonas; Cederroth, Christopher R. (1 August 2020). "Current Clinical Trials for Tinnitus". Otolaryngologic Clinics of North America. 53 (4): 651–666. doi:10.1016/j.otc.2020.03.012. ISSN 0030-6665. PMID 32334870. S2CID 216557264. Retrieved 24 February 2022.
- ^ Bhakuni, Dewan S.; Jain, Sudha; Singh, Awadhesh N. (1980). "Biosynthesis of the bisbenzylisoquinoline alkaloid, tetrandrine". Phytochemistry. 19 (11): 2347–2350. Bibcode:1980PChem..19.2347B. doi:10.1016/S0031-9422(00)91024-0.
- ^ Tetrandrine(Fanchinine) | CAS 518-34-3 | calcium channel Inhibitor | Fanchinine, Hanfangchin A, NSC 77037, S,S-(+)-Tetrandrine, Sinomenine A, TTD, Tetrandrin, d...

