Sex ratio
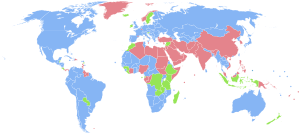
| Countries with more males than females. Countries with the same number of males and females (accounting that the ratio has 3 significant figures, i.e., 1.00 males to 1.00 females). Countries with more females than males. No data |
A sex ratio is the ratio of males to females in a population. As explained by Fisher's principle, for evolutionary reasons this is typically about 1:1 in species which reproduce sexually.[2][3] However, many species deviate from an even sex ratio, either periodically or permanently. Examples include parthenogenic and androgenetic[4] species, periodically mating organisms such as aphids, some eusocial wasps, bees, ants, and termites.[5]
The human sex ratio is of particular interest to anthropologists and demographers. In human societies, sex ratios at birth may be considerably skewed by factors such as the age of mother at birth[6] and by sex-selective abortion and infanticide. Exposure to pesticides and other environmental contaminants may be a significant contributing factor as well.[7] As of 2024, the global sex ratio at birth is estimated at 107 boys to 100 girls (1,000 boys per 934 girls).[8] By old age, the sex ratio reverses, with 81 older men for every 100 older women; across all ages, the global population is nearly balanced, with 101 males for every 100 females.[8]
Types
[edit]In most species, the sex ratio varies according to the age profile of the population.[9]
It is generally divided into four subdivisions:
- primary sex ratio — ratio at fertilization
- secondary sex ratio — ratio at birth
- tertiary sex ratio — ratio in sexually mature organisms
- The tertiary sex ratio is equivalent to the adult sex ratio (ASR), which is defined as the ratio of adult males to females in a population.[10][11]
- The operational sex ratio (OSR) is the ratio of sexually active males to females in a population, and is therefore derived from a subset of the individuals included when calculating the ASR.[11] Although conceptually distinct, researchers have sometimes equated the ASR with the OSR, particularly in experimental studies of animals where the difference between the two values may not always be readily apparent.[12]
- quaternary sex ratio — ratio in post-reproductive organisms
These definitions can be somewhat subjective since they lack clear boundaries.
Sex ratio theory
[edit]Sex ratio theory is a field of academic study which seeks to understand the sex ratios observed in nature from an evolutionary perspective. It continues to be heavily influenced by the work of Eric Charnov.[13] He defines five major questions, both for his book and the field in general (slightly abbreviated here):
- For a dioecious species, what is the equilibrium sex ratio maintained by natural selection?
- For a sequential hermaphrodite, what is the equilibrium sex order and time of sex change?
- For a simultaneous hermaphrodite, what is the equilibrium allocation of resources to male versus female function in each breeding season?
- Under what conditions are the various states of hermaphroditism or dioecy evolutionarily stable? When is a mixture of sexual types stable?
- When does selection favour the ability of an individual to alter its allocation to male versus female function, in response to particular environmental or life history situations?
Biological research mostly concerns itself with sex allocation rather than sex ratio, sex allocation denoting the allocation of energy to either sex. Common research themes are the effects of local mate and resource competition (often abbreviated LMC and LRC, respectively).
Fisher's principle
[edit]Fisher's principle (1930)[2] explains why in most species, the sex ratio is approximately 1:1. His argument was summarised by W. D. Hamilton (1967)[3] as follows, assuming that parents invest the same whether raising male or female offspring:
- Suppose male births are less common than female.
- A newborn male then has better mating prospects than a newborn female, and therefore can expect to have more offspring.
- Therefore parents genetically disposed to produce males tend to have more than average numbers of grandchildren born to them.
- Therefore the genes for male-producing tendencies spread, and male births become more common.
- As the 1:1 sex ratio is approached, the advantage associated with producing males dies away.
- The same reasoning holds if females are substituted for males throughout. Therefore 1:1 is the equilibrium ratio.
In modern language, the 1:1 ratio is the evolutionarily stable strategy (ESS).[14] This ratio has been observed in many species, including the bee Macrotera portalis. A study performed by Danforth observed no significant difference in the number of males and females from the 1:1 sex ratio.[15]
Examples in non-human species
[edit]Environmental and individual control
[edit]Spending equal amounts of resources to produce offspring of either sex is an evolutionarily stable strategy: if the general population deviates from this equilibrium by favoring one sex, one can obtain higher reproductive success with less effort by producing more of the other. For species where the cost of successfully raising one offspring is roughly the same regardless of its sex, this translates to an approximately equal sex ratio.
Bacteria of the genus Wolbachia cause skewed sex ratios in some arthropod species as they kill males. Sex-ratio of adult populations of pelagic copepods is usually skewed towards dominance of females. However, there are differences in adult sex ratios between families: in families in which females require multiple matings to keep producing eggs, sex ratios are less biased (close to 1); in families in which females can produce eggs continuously after only one mating, sex ratios are strongly skewed towards females.[16]
Several species of reptiles have temperature-dependent sex determination, where incubation temperature of eggs determines the sex of the individual. In the American alligator, for example, females are hatched from eggs incubated between 27.7 to 30 °C (81.9 to 86.0 °F), whereas males are hatched from eggs 32.2 to 33.8 °C (90.0 to 92.8 °F). In this method, however, all eggs in a clutch (20–50) will be of the same sex. In fact, the natural sex ratio of this species is five females to one male.[17]
In birds, mothers can influence the sex of their chicks. In peafowl, maternal body condition can influence the proportion of daughters in the range from 25% to 87%.[18]
Dichogamy (sequential hermaphroditism) is normal in several groups of fish, such as wrasses, parrotfish and clownfish. This can cause a discrepancy in the sex ratios as well. In the bluestreak cleaner wrasse, there is only one male for every group of 6-8 females. If the male fish dies, the strongest female changes its sex to become the male for the group. All of these wrasses are born female, and only become male in this situation. Other species, like clownfish, do this in reverse, where all start out as non-reproductive males, and the largest male becomes a female, with the second-largest male maturing to become reproductive.
Domesticated animals
[edit]Traditionally, farmers have discovered that the most economically efficient community of animals will have a large number of females and a very small number of males. A herd of cows with a few bulls or a flock of hens with one rooster are the most economical sex ratios for domesticated livestock.[citation needed]
Dioecious plants secondary sex ratio and amount of pollen
[edit]It was found that the amount of fertilizing pollen can influence secondary sex ratio in dioecious plants. Increase in pollen amount leads to decrease in number of male plants in the progeny. This relationship was confirmed on four plant species from three families – Rumex acetosa (Polygonaceae),[19][20] Melandrium album (Caryophyllaceae),[21][22] Cannabis sativa[23] and Humulus japonicus (Cannabinaceae).[24]
Polyandrous and cooperatively breeding homeotherms
[edit]In charadriiform birds, recent research has shown clearly that polyandry and sex-role reversal (where males care and females compete for mates) as found in phalaropes, jacanas, painted snipe and a few plover species is clearly related to a strongly male-biased adult sex ratio.[25] Those species with male care and polyandry invariably have adult sex ratios with a large surplus of males,[25] which in some cases can reach as high as six males per female.[26]
Male-biased adult sex ratios have also been shown to correlate with cooperative breeding in mammals such as alpine marmots and wild canids.[27] This correlation may also apply to cooperatively breeding birds,[28] though the evidence is less clear.[25] It is known, however, that both male-biased adult sex ratios[29] and cooperative breeding tend to evolve where caring for offspring is extremely difficult due to low secondary productivity, as in Australia[30] and Southern Africa. It is also known that in cooperative breeders where both sexes are philopatric like the varied sittella,[31] adult sex ratios are equally or more male-biased than in those cooperative species, such as fairy-wrens, treecreepers and the noisy miner[32] where females always disperse.
See also
[edit]- Evolution of sex
- Operational sex ratio
- Sex allocation
- Trivers–Willard hypothesis
- XY sex-determination system
Humans:
- Human sex ratio
- List of countries by sex ratio
- Bride kidnapping
- Groom kidnapping
- Demographic transition
- Sex selection
- Sex-selective abortion and infanticide
- Youth bulge
Institutions:
References
[edit]- ^ Data from the CIA World Factbook [1]. Map compiled in 2021, data from 2020.
- ^ a b Fisher RA (1930). The Genetical Theory of Natural Selection. Oxford: Clarendon Press. pp. 141–143 – via Internet Archive.
- ^ a b Hamilton WD (April 1967). "Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology". Science. 156 (3774): 477–488. Bibcode:1967Sci...156..477H. doi:10.1126/science.156.3774.477. JSTOR 1721222. PMID 6021675.
- ^ Schwander T, Oldroyd BP (28 Sep 2008). "Androgenesis: where males hijack eggs to clone themselves".
- ^ Kobayashi K, Hasegawa E, Yamamoto Y, Kawatsu K, Vargo EL, Yoshimura J, et al. (2013). "Sex ratio biases in termites provide evidence for kin selection". Nature Communications. 4: 2048. Bibcode:2013NatCo...4.2048K. doi:10.1038/ncomms3048. hdl:2123/11211. PMID 23807025.
- ^ "Trend Analysis of the sex Ratio at Birth in the United States" (PDF). U.S. Department of Health and Human Services, National Center for Health Statistics.
- ^ Davis DL, Gottlieb MB, Stampnitzky JR (April 1998). "Reduced ratio of male to female births in several industrial countries: a sentinel health indicator?". JAMA. 279 (13): 1018–1023. doi:10.1001/jama.279.13.1018. PMID 9533502.
- ^ a b "Field Listing—Sex ratio". CIA Factbook. The Central Intelligence Agency of the United States. Retrieved 2024-04-18. (2023 estimates)
- ^ Coney NS, Mackey WC (1998). "The Woman as Final Arbiter: A Case for the Facultative Character of the Human Sex Ratio". Journal of Sex Research. 35 (2): 169–175. doi:10.1080/00224499809551930.
- ^ Parker GA, Simmons LW (1996). "Parental Investment and the Control of Sexual Selection: Predicting the Direction of Sexual Competition" (PDF). Proceedings of the Royal Society B: Biological Sciences. 263 (1368): 315–321. doi:10.1098/rspb.1996.0048. JSTOR 50614. Retrieved 24 December 2022.
- ^ a b Kvarnemo C, Ahnesjö I (2002). "Operational Sex Ratios and Mating Competition" (PDF). In Hardy IC (ed.). Sex Ratios: Concepts and Research Methods (PDF). Cambridge: Cambridge University Press. pp. 366–382. doi:10.1017/CBO9780511542053.019. ISBN 9780521818964. Retrieved 24 December 2022.
- ^ Székely T, Weissing FJ, Komdeur J (August 2014). "Adult sex ratio variation: implications for breeding system evolution". Journal of Evolutionary Biology. 27 (8): 1500–1512. doi:10.1111/jeb.12415. PMID 24848871. S2CID 8350737.
- ^ Charnov EL (1982). Sex Allocation. Princeton: Princeton University Press. ISBN 9780691083124.
- ^ Maynard Smith J, Price GR (1973). "The logic of animal conflict". Nature. 246 (5427): 15–8. Bibcode:1973Natur.246...15S. doi:10.1038/246015a0. S2CID 4224989.
- ^ Danforth B (1991). "Female Foraging and Intranest Behavior of a Communal Bee, Perdita portalis (Hymenoptera: Andrenidae)". Annals of the Entomological Society of America. 84 (5): 537–548. doi:10.1093/aesa/84.5.537.
- ^ Kiørboe T (May 2006). "Sex, sex-ratios, and the dynamics of pelagic copepod populations". Oecologia. 148 (1): 40–50. Bibcode:2006Oecol.148...40K. doi:10.1007/s00442-005-0346-3. PMID 16425044. S2CID 13412222.
- ^ Ferguson MW, Joanen T (April 1982). "Temperature of egg incubation determines sex in Alligator mississippiensis". Nature. 296 (5860): 850–853. Bibcode:1982Natur.296..850F. doi:10.1038/296850a0. PMID 7070524. S2CID 4307265.
- ^ Pike TW, Petrie M (October 2005). "Maternal body condition and plasma hormones affect offspring sex ration in peafowl". Animal Behaviour. 70 (4): 745–51. doi:10.1016/j.anbehav.2004.12.020. S2CID 53185717.
- ^ Correns C (1922). "Geschlechtsbestimmung und Zahlenverhaltnis der Geschlechter beim Sauerampfer (Rumex acetosa)". Biologisches Zentralblatt. 42: 465–80.
- ^ Rychlewski JE, Zarzycki K (1975). "Sex ratio in seeds of Rumex acetosa L. as a result of sparse or abundant pollination". Acta Biol Crac Ser Bot. 18: 101–14.
- ^ Correns C (1928). "Bestimmung, Vererbung und Verteilung des Geschlechter bei den hoheren Pflanzen". Handb. Vererbungswiss. 2: 1–138.
- ^ Mulcahy DL (1967). "Optimal sex ratio in Silene alba". Heredity. 22 (3): 411–423. doi:10.1038/hdy.1967.50.
- ^ Riede W (January 1925). "Beiträge zum Geschlechts-und Anpassungsproblem". Flora oder Allgemeine Botanische Zeitung. 118: 421–452. doi:10.1016/S0367-1615(17)32904-X.
- ^ Kihara H, Hirayoshi J (1932). "Die Geschlechtschromosomen von Humulus japonicus. Sieb. et. Zuce". 8th Congr. Jap. Ass. Adv. Sci.: 363–367. (cit.: Plant Breeding Abstr., 1934, 5, № 3, p. 248, ref. № 768).
- ^ a b c Liker A, Freckleton RP, Székely T (2013). "The evolution of sex roles in birds is related to adult sex ratio". Nature Communications. 4: 1587. Bibcode:2013NatCo...4.1587L. doi:10.1038/ncomms2600. PMID 23481395.
- ^ Kosztolányi A, Barta Z, Küpper C, Székely T (August 2011). "Persistence of an extreme male-biased adult sex ratio in a natural population of polyandrous bird". Journal of Evolutionary Biology. 24 (8): 1842–1846. doi:10.1111/j.1420-9101.2011.02305.x. PMID 21749544. S2CID 6954828.
- ^ Allainé, Dominique; Brondex, Francine; Graziani, Laurent; Coulon, Jacques and Till-Bottraud, Irène; "Male-biased sex ratio in litters of alpine marmots supports the helper repayment hypothesis"
- ^ Doerr ED, Doerr VA (2006). "Comparative demography of treecreepers: evaluating hypotheses for the evolution and maintenance of cooperative breeding". Animal Behaviour. 72 (1): 147–159. doi:10.1016/j.anbehav.2005.10.017. S2CID 53165151.
- ^ Kokko H, Jennions MD (July 2008). "Parental investment, sexual selection and sex ratios". Journal of Evolutionary Biology. 21 (4): 919–948. doi:10.1111/j.1420-9101.2008.01540.x. hdl:1885/54578. PMID 18462318. S2CID 14624385.
- ^ Orians GH, Milewski AV (August 2007). "Ecology of Australia: the effects of nutrient-poor soils and intense fires". Biological Reviews of the Cambridge Philosophical Society. 82 (3): 393–423. doi:10.1111/j.1469-185x.2007.00017.x. PMID 17624961. S2CID 39566226.
- ^ Noske RA (1986). "Intersexual niche segregation among three bark-foraging birds of eucalypt forests". Australian Journal of Ecology. 11 (3): 255–267. doi:10.1111/j.1442-9993.1986.tb01396.x.
- ^ Arnold KE, Griffith SC, Goldizen AW (2001). "Sex-biased hatching sequences in the cooperatively breeding noisy miner". Journal of Avian Biology. 32 (3): 219–223. doi:10.1111/j.0908-8857.2001.320303.x.
Further reading
[edit]- Coale AJ, Banister J (December 1996). "Five decades of missing females in China". Proceedings of the American Philosophical Society. 140 (4): 421–450. JSTOR 987286. Also printed as Coale AJ, Banister J (August 1994). "Five decades of missing females in China". Demography. 31 (3): 459–479. doi:10.2307/2061752. JSTOR 2061752. PMID 7828766. S2CID 24724998.
- Nishimura K, Jahn GC (1996). "Sex allocation of three solitary ectoparasitic wasp species on bean weevil larvae: sex ratio change with host quality and local mate competition". Journal of Ethology. 14 (1): 27–34. doi:10.1007/BF02350089. S2CID 10590797.
- Trivers RL, Willard DE (January 1973). "Natural selection of parental ability to vary the sex ratio of offspring". Science. 179 (4068): 90–92. Bibcode:1973Sci...179...90T. doi:10.1126/science.179.4068.90. PMID 4682135. S2CID 29326420.
- Rath RM, Mishra AK (2005). Techniques for Sex Ratio Analysis. Association of Professional Geographers.

