Amastatin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.131.532 |
| Chemical and physical data | |
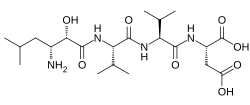
| Formula | C21H38N4O8 |
| Molar mass | 474.555 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Amastatin, also known as 3-amino-2-hydroxy-5-methylhexanoyl-L-valyl-L-valyl-L-aspartic acid, is a naturally occurring, competitive and reversible aminopeptidase inhibitor that was isolated from Streptomyces sp. ME 98-M3.[1] It specifically inhibits leucyl aminopeptidase, alanyl aminopeptidase (aminopeptidase M/N), bacterial leucyl aminopeptidase (Aeromonas proteolytica aminopeptidase), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase),[2] and, to a lesser extent, glutamyl aminopeptidase (aminopeptidase A),[3] as well as other aminopeptidases.[4] It does not inhibit arginyl aminopeptidase (aminopeptidase B).[5][6] Amastatin has been found to potentiate the central nervous system effects of oxytocin and vasopressin in vivo.[7] It also inhibits the degradation of met-enkephalin, dynorphin A, and other endogenous peptides.[8]
See also
[edit]References
[edit]- ^ Buckingham J (2 December 1993). "Amastatins". Dictionary of Natural Products. CRC Press. pp. 197–. ISBN 978-0-412-46620-5.
- ^ Nakanishi Y, Nomura S, Okada M, Ito T, Katsumata Y, Kikkawa F, et al. (September 2000). "Immunoaffinity purification and characterization of native placental leucine aminopeptidase/oxytocinase from human placenta". Placenta. 21 (7): 628–634. doi:10.1053/plac.2000.0564. PMID 10985965.
- ^ Schloss JV (31 July 1989). "Modern Aspects of Enzyme Inhibition with Particular Emphasis on Reaction-Intermediate Analogs and Other Potent, Reversible Inhibitors". In Boger P, Sandmann G (eds.). Target Sites of Herbicide Action. CRC Press. pp. 203–. ISBN 978-0-8493-4985-0.
- ^ Scott T, Mercer EI (1997). Concise Encyclopedia Biochemistry and Molecular Biology. Walter de Gruyter. pp. 35–. ISBN 978-3-11-014535-9.
- ^ Umezawa H (9 May 2014). Small Molecular Immunomodifiers of Microbial Origin: Fundamental and Clinical Studies of Bestatin. Elsevier Science. pp. 10–. ISBN 978-1-4831-9033-4.
- ^ Drey CN (6 December 2012). "Beta and Higher Homologous Amino Acids". In Barrett G (ed.). Chemistry and Biochemistry of the Amino Acids. Springer Science & Business Media. pp. 28–. doi:10.1007/978-94-009-4832-7_3. ISBN 978-94-009-4832-7.
- ^ Meisenberg G, Simmons WH (1984). "Amastatin potentiates the behavioral effects of vasopressin and oxytocin in mice". Peptides. 5 (3): 535–539. doi:10.1016/0196-9781(84)90083-4. PMID 6540873. S2CID 3881661.
- ^ Oka T, Hiranuma T, Liu XF, Ohgiya N, Iwao K, Matsumiya T (April 1993). "[Enkephalin-inactivating enzymes]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 101 (4): 197–207. doi:10.1254/fpj.101.4_197. PMID 8390390.

