2C-T-3
Appearance
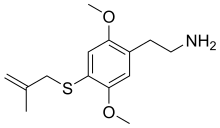
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-{2,5-Dimethoxy-4-[(2-methylprop-2-en-1-yl)sulfanyl]phenyl}ethan-1-amine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H21NO2S | |
| Molar mass | 267.39 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2C-T-3 (also initially numbered as 2C-T-20) is a lesser-known psychedelic drug related to compounds such as 2C-T-7 and 2C-T-16. It was named by Alexander Shulgin but was never made or tested by him, and was instead first synthesised by Daniel Trachsel some years later. It has a binding affinity of 11nM at 5-HT2A and 40nM at 5-HT2C. It is reportedly a potent psychedelic drug with an active dose in the 15–40 mg range, and a duration of action of 8–14 hours, with visual effects comparable to related drugs such as methallylescaline.[1][2][3]
See also
[edit]References
[edit]- ^ Trachsel D (2003). "Synthesis of novel (phenylalkyl)amines for the investigation of structure-activity relationships. Part 2. 4-Thio-substituted [2-(2,5-dimethoxyphenyl)ethyl]amines (=2,5-dimethoxybenzeneethanamines)". Helvetica Chimica Acta. 86 (7): 2610–2619. doi:10.1002/hlca.200390210.
- ^ Luethi D, Trachsel D, Hoener MC, Liechti ME (May 2018). "Monoamine receptor interaction profiles of 4-thio-substituted phenethylamines (2C-T drugs)" (PDF). Neuropharmacology. 134 (Pt A): 141–148. doi:10.1016/j.neuropharm.2017.07.012. PMID 28720478. S2CID 7135811.
- ^ Trachsel D, Lehmann D, Enzensperger C (2013). Phenethylamine: Von der Struktur zur Funktion. Nachtschatten Verlag AG. pp. 788–794. ISBN 978-3-03788-700-4.

