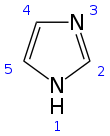
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution.
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1H-Imidazole[1] | |||
| Other names
1,3-Diazole
Glyoxaline (archaic) | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 103853 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.473 | ||
| EC Number |
| ||
| 1417 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3263 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H4N2 | |||
| Molar mass | 68.077 g/mol | ||
| Appearance | White or pale yellow solid | ||
| Density | 1.23 g/cm3, solid | ||
| Melting point | 89 to 91 °C (192 to 196 °F; 362 to 364 K) | ||
| Boiling point | 256 °C (493 °F; 529 K) | ||
| 633 g/L | |||
| Acidity (pKa) | 6.95 (for the conjugate acid) [2] | ||
| UV-vis (λmax) | 206 nm | ||
| Structure | |||
| Monoclinic | |||
| Planar 5-membered ring | |||
| 3.61 D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Corrosive | ||
| GHS labelling:[4] | |||
  
| |||
| Danger | |||
| H302, H314, H360D | |||
| P263, P270, P280, P301+P310, P305+P351+P338, P308+P313[3] | |||
| Flash point | 146 °C (295 °F; 419 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Many natural products, especially alkaloids, contain the imidazole ring. These imidazoles share the 1,3-C3N2 ring but feature varied substituents. This ring system is present in important biological building blocks, such as histidine and the related hormone histamine. Many drugs contain an imidazole ring, such as certain antifungal drugs, the nitroimidazole series of antibiotics, and the sedative midazolam.[5][6][7][8][9]
When fused to a pyrimidine ring, it forms a purine, which is the most widely occurring nitrogen-containing heterocycle in nature.[10]
The name "imidazole" was coined in 1887 by the German chemist Arthur Rudolf Hantzsch (1857–1935).[11]
Structure and properties
editImidazole is a planar 5-membered ring, that exists in two equivalent tautomeric forms because hydrogen can be bound to one or another nitrogen atom. Imidazole is a highly polar compound, as evidenced by its electric dipole moment of 3.67 D,[12] and is highly soluble in water. The compound is classified as aromatic due to the presence of a planar ring containing 6 π-electrons (a pair of electrons from the protonated nitrogen atom and one from each of the remaining four atoms of the ring). Some resonance structures of imidazole are shown below:
Amphoterism
editImidazole is amphoteric, which is to say that it can function both as an acid and as a base. As an acid, the pKa of imidazole is 14.5, making it less acidic than carboxylic acids, phenols, and imides, but slightly more acidic than alcohols. The acidic proton is the one bound to nitrogen. Deprotonation gives the imidazolide anion, which is symmetrical. As a base, the pKa of the conjugate acid (cited as pKBH+ to avoid confusion between the two) is approximately 7, making imidazole approximately sixty times more basic than pyridine. The basic site is the nitrogen with the lone pair (and not bound to hydrogen). Protonation gives the imidazolium cation, which is symmetrical.
Preparation
editImidazole was first reported in 1858 by the German chemist Heinrich Debus, although various imidazole derivatives had been discovered as early as the 1840s. It was shown that glyoxal, formaldehyde, and ammonia condense to form imidazole (glyoxaline, as it was originally named).[13] This synthesis, while producing relatively low yields, is still used for generating C-substituted imidazoles.
In one microwave modification, the reactants are benzil, benzaldehyde and ammonia in glacial acetic acid, forming 2,4,5-triphenylimidazole ("lophine").[14]
Imidazole can be synthesized by numerous methods besides the Debus method. Many of these syntheses can also be applied to different substituted imidazoles and imidazole derivatives by varying the functional groups on the reactants. These methods are commonly categorized by which and how many bonds form to make the imidazole rings. For example, the Debus method forms the (1,2), (3,4), and (1,5) bonds in imidazole, using each reactant as a fragment of the ring, and thus this method would be a three-bond-forming synthesis. A small sampling of these methods is presented below.
Formation of one bond
editThe (1,5) or (3,4) bond can be formed by the reaction of an imidate and an α-aminoaldehyde or α-aminoacetal. The example below applies to imidazole when R1 = R2 = hydrogen.
Formation of two bonds
editThe (1,2) and (2,3) bonds can be formed by treating a 1,2-diaminoalkane, at high temperatures, with an alcohol, aldehyde, or carboxylic acid. A dehydrogenating catalyst, such as platinum on alumina, is required.
The (1,2) and (3,4) bonds can also be formed from N-substituted α-aminoketones and formamide with heat. The product will be a 1,4-disubstituted imidazole, but here since R1 = R2 = hydrogen, imidazole itself is the product. The yield of this reaction is moderate, but it seems to be the most effective method of making the 1,4 substitution.
Formation of four bonds
editThis is a general method that is able to give good yields for substituted imidazoles. In essence, it is an adaptation of the Debus method called the Debus-Radziszewski imidazole synthesis. The starting materials are substituted glyoxal, aldehyde, amine, and ammonia or an ammonium salt.[15]
Formation from other heterocycles
editImidazole can be synthesized by the photolysis of 1-vinyltetrazole. This reaction will give substantial yields only if the 1-vinyltetrazole is made efficiently from an organotin compound, such as 2-tributylstannyltetrazole. The reaction, shown below, produces imidazole when R1 = R2 = R3 = hydrogen.
Imidazole can also be formed in a vapor-phase reaction. The reaction occurs with formamide, ethylenediamine, and hydrogen over platinum on alumina, and it must take place between 340 and 480 °C. This forms a very pure imidazole product.
- Van Leusen reaction[16]
The Van Leusen reaction can also be employed to form imidazoles starting from TosMIC and an aldimine. The Van Leusen Imidazole Synthesis allows the preparation of imidazoles from aldimines by reaction with tosylmethyl isocyanide (TosMIC). The reaction has later been expanded to a two-step synthesis in which the aldimine is generated in situ: the Van Leusen Three-Component Reaction (vL-3CR).
Biological significance and applications
editImidazole is incorporated into many important biological compounds. The most pervasive is the amino acid histidine, which has an imidazole side-chain. Histidine is present in many proteins and enzymes, e.g. by binding metal cofactors, as seen in hemoglobin.
Imidazole-based histidine compounds play a very important role in intracellular buffering.[17] Histidine can be decarboxylated to histamine. Histamine can cause urticaria (hives) when it is produced during allergic reaction.
Imidazole substituents are found in many pharmaceuticals. Synthetic imidazoles are present in many fungicides and antifungal, antiprotozoal, and antihypertensive medications. Imidazole is part of the theophylline molecule, found in tea leaves and coffee beans, that stimulates the central nervous system. It is present in the anticancer medication mercaptopurine, which combats leukemia by interfering with DNA activities.
A number of substituted imidazoles, including clotrimazole, are selective inhibitors of nitric oxide synthase, which makes them interesting drug targets in inflammation, neurodegenerative diseases and tumors of the nervous system.[18][19] Other biological activities of the imidazole pharmacophore relate to the downregulation of intracellular Ca2+ and K+ fluxes, and interference with translation initiation.[20]
Pharmaceutical derivatives
editThe substituted imidazole derivatives are valuable in treatment of many systemic fungal infections.[21] Imidazoles belong to the class of azole antifungals, which includes ketoconazole, miconazole, and clotrimazole.
For comparison, another group of azoles is the triazoles, which includes fluconazole, itraconazole, and voriconazole. The difference between the imidazoles and the triazoles involves the mechanism of inhibition of the cytochrome P450 enzyme. The N3 of the imidazole compound binds to the heme iron atom of ferric cytochrome P450, whereas the N4 of the triazoles bind to the heme group. The triazoles have been shown to have a higher specificity for the cytochrome P450 than imidazoles, thereby making them more potent than the imidazoles.[22]
Some imidazole derivatives show effects on insects, for example sulconazole nitrate exhibits a strong anti-feeding effect on the keratin-digesting Australian carpet beetle larvae Anthrenocerus australis, as does econazole nitrate with the common clothes moth Tineola bisselliella.[23]
Industrial applications
editImidazole itself has few direct applications. It is instead a precursor to a variety of agrichemicals, including enilconazole, climbazole, clotrimazole, prochloraz, and bifonazole.[24]
Coordination chemistry
editImidazole and its derivatives have high affinity for metal cations. One of the applications of imidazole is in the purification of His-tagged proteins in immobilised metal affinity chromatography (IMAC). Imidazole is used to elute tagged proteins bound to nickel ions attached to the surface of beads in the chromatography column. An excess of imidazole is passed through the column, which displaces the His-tag from nickel coordination, freeing the His-tagged proteins.
Use in biological research
editImidazole is a suitable buffer for pH 6.2 to 7.8,.[25] Pure imidazole has essentially no absorbance at protein relevant wavelengths (280 nm),[26][27] however lower purities of imidazole can give notable absorbance at 280 nm. Imidazole can interfere with the Lowry protein assay.[28]
Imidazole is often used in protein purification, where recombinant proteins with polyhistidine tags are immobilized onto nickel resins and eluted with a high imidazole concentration.
Salts of imidazole
editSalts of imidazole where the imidazole ring is the cation are known as imidazolium salts (for example, imidazolium chloride or nitrate).[29] These salts are formed from the protonation or substitution at nitrogen of imidazole. These salts have been used as ionic liquids and precursors to stable carbenes. Salts where a deprotonated imidazole is an anion are also well known; these salts are known as imidazolates (for example, sodium imidazolate, NaC3H3N2).
Related heterocycles
edit- Benzimidazole, an analog with a fused benzene ring
- Dihydroimidazole or imidazoline, an analog where the 4,5-double bond is saturated
- Pyrrole, an analog with only one nitrogen atom in position 1
- Oxazole, an analog with the nitrogen atom in position 1 replaced by oxygen
- Thiazole, an analog with the nitrogen atom in position 1 replaced by sulfur
- Pyrazole, an analog with two adjacent nitrogen atoms
- Triazoles, analogs with three nitrogen atoms
Safety
editImidazole has low acute toxicity as indicated by the LD50 of 970 mg/kg (Rat, oral).[24]
See also
edit- 1-Methylimidazole
- 4-Methylimidazole
- Imidazoline (dihydroimidazole)
References
edit- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 140. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Walba, H.; Isensee, R. W. (1961). "Acidity constants of some arylimidazoles and their cations". J. Org. Chem. 26 (8): 2789–2791. doi:10.1021/jo01066a039.
- ^ "Imidazole". molekula.com. Molekula Group. Archived from the original on 2018-10-19. Retrieved 2018-10-19.
- ^ "Imidazole". pubchem.ncbi.nlm.nih.gov. Archived from the original on 10 May 2023. Retrieved 17 February 2024.
- ^ Karitzky, A. R.; Rees, C.W.R.; Scriven, E.F.V. (1984). Comprehensive Heterocyclic Chemistry. Vol. 5. pp. 469–498. ISBN 978-0-08-042072-1.
- ^ Grimmett, M. Ross (1997). Imidazole and Benzimidazole Synthesis. Academic Press. ISBN 978-0-08-053445-9.
- ^ Brown, E. G. (1998). Ring Nitrogen and Key Biomolecules. Kluwer Academic Press. ISBN 978-94-011-4906-8.
- ^ Pozharskii, A. F.; et al. (1997). Heterocycles in Life and Society. John Wiley & Sons. ISBN 978-0-471-96033-1.
- ^ Gilchrist, T. L. (1985). Heterocyclic Chemistry. Bath Press. ISBN 978-0-582-01421-3.
- ^ Rosemeyer, H. (2004). "The Chemodiversity of Purine as a Constituent of Natural Products". Chemistry & Biodiversity. 1 (3): 361–401. doi:10.1002/cbdv.200490033. PMID 17191854. S2CID 12416667.
- ^ Hantzsch, A. and Weber, J. H. (1887) "Ueber Verbindungen des Thiazols (Pyridins der Thiophenreihe)" Archived 2020-05-30 at the Wayback Machine (On compounds of thiazole (pyridines of the thiophene series), Berichte der deutschen chemischen Gesellschaft, 20 : 3118–3132, see p. 3119. See also: Hantzsch, A. (1888) "Allegemeine Bemerkungen über Azole" Archived 2020-05-30 at the Wayback Machine (General observations about azoles), Annalen der Chemie, 249 : 1–6. Hantzsch proposed a reform of the nomenclature of azole compounds, including a proposal to call the heterocyclic ring C3H3(NH)N "imidazole"; see pp. 2 and 4.
- ^ Christen, Dines; Griffiths, John H.; Sheridan, John (1981). "The Microwave Spectrum of Imidazole; Complete Structure and the Electron Distribution from Nuclear Quadrupole Coupling Tensors and Dipole Moment Orientation". Zeitschrift für Naturforschung A. 36 (12): 1378–1385. Bibcode:1981ZNatA..36.1378C. doi:10.1515/zna-1981-1220. S2CID 3522351.
- ^ Debus, Heinrich (1858). "Ueber die Einwirkung des Ammoniaks auf Glyoxal" [On the reaction of ammonia upon glyoxal]. Annalen der Chemie und Pharmacie. 107 (2): 199–208. doi:10.1002/jlac.18581070209. Archived from the original on 2020-05-30. Retrieved 2016-10-01. From p. 205: "Die gereinigte Substanz stellt das oxalsaure Salz einer Basis dar, die ich mit Glyoxalin bezeichenen werde." (The purified substance constitutes the oxalic salt of a base, which I will designate as "glyoxaline".)
- ^ Crouch, R. David; Howard, Jessica L.; Zile, Jennifer L.; Barker, Kathryn H. (2006). "Microwave-Mediated Synthesis of Lophine: Developing a Mechanism To Explain a Product". J. Chem. Educ. 83 (11): 1658. Bibcode:2006JChEd..83.1658C. doi:10.1021/ed083p1658.
- ^ US patent 6,177,575, Arduengo, A. J., "Process for Manufacture of Imidazoles", issued 2001-01-23
- ^ Van Leusen, Albert M.; Wildeman, Jurjen; Oldenziel, Otto H. (1977). "Chemistry of sulfonylmethyl isocyanides. 12. Base-induced cycloaddition of sulfonylmethyl isocyanides to carbon, nitrogen double bonds. Synthesis of 1,5-disubstituted and 1,4,5-trisubstituted imidazoles from aldimines and imidoyl chlorides". Journal of Organic Chemistry. 42 (7): 1153–1159. Bibcode:1977JOrgC..42.1153A. doi:10.1021/jo00427a012.
- ^ Hochachka, P. W.; Somero, G. N. (2002). Biochemical Adaptation: Mechanisms and Process in Physiological Evolution. New York: Oxford University Press.
- ^ Castaño, T.; Encinas, A.; Pérez, C.; Castro, A.; Campillo, N. E.; Gil, C. (2008). "Design, synthesis, and evaluation of potential inhibitors of nitric oxide synthase" (PDF). Bioorg. Med. Chem. (Submitted manuscript). 16 (11): 6193–6206. doi:10.1016/j.bmc.2008.04.036. hdl:10261/87090. PMID 18477512. Archived (PDF) from the original on 2023-03-06. Retrieved 2018-07-24.
- ^ Bogle, R. G.; Whitley, G. S.; Soo, S. C.; Johnstone, A. P.; Vallance, P. (1994). "Effect of anti-fungal imidazoles on mRNA levels and enzyme activity of inducible nitric oxide synthase". Br. J. Pharmacol. 111 (4): 1257–1261. doi:10.1111/j.1476-5381.1994.tb14881.x. PMC 1910171. PMID 7518297.
- ^ Khalid, M. H.; Tokunaga, Y.; Caputy, A. J.; Walters, E. (2005). "Inhibition of tumor growth and prolonged survival of rats with intracranial gliomas following administration of clotrimazole". J. Neurosurg. 103 (1): 79–86. doi:10.3171/jns.2005.103.1.0079. PMID 16121977.
- ^ Leon Shargel (2007). Comprehensive Pharmacy Review (6th ed.). Lippincott Williams & Wilkins. p. 930. ISBN 9780781765619.
- ^ Davis, Jennifer L.; Papich, Mark G.; Heit, Mark C. (2009). "Chapter 39: Antifungal and Antiviral Drugs". In Riviere, Jim E.; Papich, Mark G. (eds.). Veterinary Pharmacology and Therapeutics (9th ed.). Wiley-Blackwell. pp. 1019–1020. ISBN 978-0-8138-2061-3.
- ^ Sunderland, M. R.; Cruickshank, R. H.; Leighs, S. J. (2014). "The efficacy of antifungal azole and antiprotozoal compounds in protection of wool from keratin-digesting insect larvae". Textile Res. J. 84 (9): 924–931. doi:10.1177/0040517513515312. S2CID 135799368.
- ^ a b Ebel, K., Koehler, H., Gamer, A. O., & Jäckh, R. (2002). "Imidazole and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_661. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - ^ Dawson, R. M. C. (1986). Data for biochemical research. Oxford: Clarendon Press. p. 325. ISBN 978-0-19-855299-4. OCLC 11865673.
- ^ "1H-Imidazole". Archived from the original on 25 April 2021. Retrieved 13 May 2021.
- ^ "Optimizing Purification of Histidine-Tagged Proteins". Archived from the original on 13 May 2021. Retrieved 13 May 2021.
- ^ Molina, F; Rueda, A; Bosque-Sendra, J.M; Megias, L (1996). "Determination of proteins in the presence of imidazole buffers". Journal of Pharmaceutical and Biomedical Analysis. 14 (3). Elsevier BV: 273–280. doi:10.1016/0731-7085(95)01615-5. ISSN 0731-7085. PMID 8851751.
- ^ Zolfigol, Mohammad A.; Khazaei, Ardeshir; Moosavi-Zare, Ahmad R.; Zare, Abdolkarim; Kruger, Hendrik G.; Asgari, Zhila; Khakyzadeh, Vahid; Kazem-Rostami, Masoud (2012-04-06). "Design of Ionic Liquid 3-Methyl-1-sulfonic Acid Imidazolium Nitrate as Reagent for the Nitration of Aromatic Compounds by in Situ Generation of NO2 in Acidic Media". The Journal of Organic Chemistry. 77 (7): 3640–3645. doi:10.1021/jo300137w. ISSN 0022-3263. PMID 22409592.