Ezetimibe, sold under the brand name Zetia among others, is a medication used to treat high blood cholesterol and certain other lipid abnormalities.[3][4] Generally it is used together with dietary changes and a statin.[5] Alone, it is less preferred than a statin.[4] It is taken by mouth.[4] It is also available in the fixed-dose combinations ezetimibe/simvastatin,[6] ezetimibe/atorvastatin,[7] ezetimibe/rosuvastatin,[4][8] and ezetimibe/bempedoic acid.[9]
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɛˈzɛtɪmɪb, -maɪb/ |
| Trade names | Zetia, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603015 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Cholesterol absorption inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 35% to 65% |
| Protein binding | >90% |
| Metabolism | Intestinal wall, liver |
| Elimination half-life | 19 h to 30 h |
| Excretion | Kidney 11%, fecal 78% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.996 |
| Chemical and physical data | |
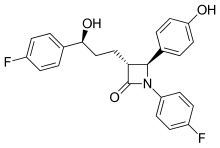
| Formula | C24H21F2NO3 |
| Molar mass | 409.433 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 164 to 166 °C (327 to 331 °F) |
| |
| |
| (verify) | |
The most commonly reported adverse events include upper respiratory tract infections, joint pain, diarrhea, and tiredness.[4] Serious side effects may include anaphylaxis, liver problems, depression, and muscle breakdown.[4][5] Use in pregnancy and breastfeeding is of unclear safety.[10] Ezetimibe works by decreasing cholesterol absorption in the intestines.[5]
Ezetimibe was approved for medical use in the United States in 2002.[4] It is available as a generic medication.[5] In 2022, it was the 79th most commonly prescribed medication in the United States, with more than 8 million prescriptions.[11][12]
Medical uses
editAdding ezetimibe to statin treatment of high blood cholesterol has no effect on overall mortality or cardiovascular mortality, although it significantly reduces the risk of myocardial infarction and stroke.[13] Combining ezetimibe with simvastatin had no effect on overall mortality but did lower the risk of heart attack or stroke in people with prior heart attack.[14][15] Several treatment guidelines recommend adding ezetimibe in select high risk persons in whom LDL goals cannot be achieved by maximally tolerated statin alone.[16][17][18][19][20]
Initiation of ezetimibe along with high-intensity statin therapy at the time of an acute coronary syndrome (ACS) event was associated with significantly better cholesterol reduction at day-7, 1-month, 3-months and 1-year post ACS event; which translated into significantly lower recurrent cardiovascular events (death from any cause, major ACS, non-fatal stroke, non-fatal myocardial infarction, and ischemic stroke) post the index event of ACS. [21]
Ezetimibe is indicated in the United States as an add-on to dietary measures to reduce levels of certain lipids in people with:[3]
- Primary hyperlipidemia, alone or with a statin
- Mixed hyperlipidemia, in combination with fenofibrate
- Homozygous familial hypercholesterolemia, in combination with specific statins
- Homozygous sitosterolemia
A 2018 review found that ezetimibe used as sole treatment slightly lowered plasma levels of lipoprotein(a), but the effect was not large enough to be important.[22]
Ezetimibe improves the non-alcoholic fatty liver disease activity score but the available evidence indicates it does not improve outcomes of hepatic steatosis.[23]
Contraindications
editThe two contraindications to taking ezetimibe are a previous allergic reaction to it, including symptoms of rash, angioedema, and anaphylaxis, and severe liver disease, especially when taken with a statin.[24]
Ezetimibe may have significant medication interactions with ciclosporin and with fibrates other than fenofibrate.[3]
Adverse effects
editCommon adverse drug reactions (≥1% of patients) associated with ezetimibe therapy include headache and/or diarrhea (steatorrhea). Infrequent adverse effects (0.1–1% of patients) include myalgia and/or raised liver function test (ALT/AST) results. Rarely (<0.1% of patients), hypersensitivity reactions (rash, angioedema) or myopathy may occur.[3] Cases of muscle problems (myalgia and rhabdomyolysis) have been reported and are included as warnings on the label for ezetimibe.[3]
Since NPC1L1 also regulates vitamin K uptake, the use of ezetimibe can lead to side effects in warfarin therapy.[25]
Overdose
editThe incidence of overdose with ezetimibe is rare; subsequently, few data exist on the effects of overdose. However, an acute overdose of ezetimibe is expected to produce an exaggeration of its usual effects, leading to loose stools, abdominal pain, and fatigue.[26]
Pharmacology
editMechanism of action
editEzetimibe inhibits the absorption of cholesterol from the small intestine and decreases the amount of cholesterol normally available to liver cells. The lower levels of cholesterol in the liver cells leads them to absorb more cholesterol from circulation and thus lowering the levels of circulating cholesterol. It blocks the critical mediator of cholesterol absorption, the Niemann-Pick C1-like 1 (NPC1L1) protein on the gastrointestinal tract epithelial cells, as well as in hepatocytes; it blocks aminopeptidase N and interrupts a caveolin 1–annexin A2 complex involved in trafficking cholesterol.[14]
Pharmacokinetics
editWithin 4–12 hours of the oral administration of a 10-mg dose to fasting adults, the attained mean ezetimibe peak plasma concentration (Cmax) was 3.4–5.5 ng/ml. Following oral administration, ezetimibe is absorbed and extensively conjugated to a phenolic glucuronide (active metabolite). Mean Cmax (45–71 ng/ml) of ezetimibe-glucuronide is attained within 1–2 hours. The concomitant administration of food (high-fat vs. nonfat meals) has no effect on the extent of absorption of ezetimibe. However, coadministration with a high-fat meal increases its Cmax by 38%. The absolute bioavailability cannot be determined, since ezetimibe is insoluble in aqueous media suitable for injection. Ezetimibe and its active metabolites are highly bound to human plasma proteins (90%).[3]
Ezetimibe is primarily metabolized in the liver and the small intestine via glucuronide conjugation with subsequent renal and biliary excretion.[27] Both the parent compound and its active metabolite are eliminated from plasma with a half-life around 22 hours, allowing for once-daily dosing. Ezetimibe lacks significant inhibitor or inducer effects on cytochrome P450 isoenzymes, which explains its limited number of drug interactions. No dose adjustment is needed in patients with chronic kidney disease or mild hepatic dysfunction (Child-Pugh score 5–6). Due to insufficient data, the manufacturer does not recommend ezetimibe for patients with moderate to severe hepatic impairment (Child-Pugh score 7–15). In patients with mild, moderate, or severe hepatic impairment, the mean AUC values for total ezetimibe are increased about 1.7-fold, 3-to-4-fold, and 5-to-6-fold, respectively, compared to healthy subjects.[3]
References
edit- ^ a b "AusPAR: Ezetimibe". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 20 April 2024.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b c d e f g h "Zetia- ezetimibe tablet". DailyMed. 26 January 2011. Archived from the original on 10 May 2021. Retrieved 13 August 2022.
- ^ a b c d e f g "Ezetimibe Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 17 June 2019. Retrieved 13 April 2019.
- ^ a b c d British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 196. ISBN 9780857113382.
- ^ "Vytorin- ezetimibe and simvastatin tablet". DailyMed. 1 June 2022. Archived from the original on 14 August 2022. Retrieved 13 August 2022.
- ^ "Liptruzet (ezetimibe and atorvastatin) tablets for oral useInitial U.S. Approval: 2013". DailyMed. 30 September 2016. Archived from the original on 14 August 2022. Retrieved 13 August 2022.
- ^ "Roszet- rosuvastatin and ezetimibe tablet Roszet (- rosuvastatin and ezetimibe tablet". DailyMed. 15 September 2021. Archived from the original on 14 August 2022. Retrieved 13 August 2022.
- ^ "Nexlizet- bempedoic acid and ezetimibe tablet, film coated". DailyMed. 24 September 2021. Retrieved 13 August 2022.
- ^ "Ezetimibe (Zetia) Use During Pregnancy". Drugs.com. Archived from the original on 13 April 2019. Retrieved 13 April 2019.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Ezetimibe Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ Savarese G, De Ferrari GM, Rosano GM, Perrone-Filardi P (December 2015). "Safety and efficacy of ezetimibe: A meta-analysis". International Journal of Cardiology. 201: 247–252. doi:10.1016/j.ijcard.2015.08.103. PMID 26301648.
- ^ a b Phan BA, Dayspring TD, Toth PP (2012). "Ezetimibe therapy: mechanism of action and clinical update". Vascular Health and Risk Management. 8: 415–427. doi:10.2147/VHRM.S33664. PMC 3402055. PMID 22910633.
- ^ Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. (June 2015). "Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes". The New England Journal of Medicine. 372 (25): 2387–2397. doi:10.1056/NEJMoa1410489. hdl:11573/1150638. PMID 26039521.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Alenghat FJ, Davis AM (February 2019). "Management of Blood Cholesterol". JAMA. 321 (8): 800–801. doi:10.1001/jama.2019.0015. PMC 6679800. PMID 30715135.
- ^ Catapano AL, Reiner Z, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. (July 2011). "ESC/EAS Guidelines for the management of dyslipidaemias The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS)". Atherosclerosis. 217 (1): 3–46. doi:10.1016/j.atherosclerosis.2011.06.011. PMID 21882396.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, et al. (2013). "Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version". Journal of Atherosclerosis and Thrombosis. 20 (6): 517–523. doi:10.5551/jat.15792. PMID 23665881.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ "Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease | Guidance and guidelines | NICE". Archived from the original on 19 November 2014.
- ^ Grundy SM, Arai H, Barter P, Bersot TP, Betteridge DJ, Carmena R, et al. (Expert Dyslipidemia Panel of the International Atherosclerosis Society) (2014). "An International Atherosclerosis Society Position Paper: global recommendations for the management of dyslipidemia--full report". Journal of Clinical Lipidology. 8 (1): 29–60. doi:10.1016/j.jacl.2013.12.005. PMID 24528685.
- ^ Mahajan K, Nagendra L, Dhall A, Dutta D (February 2024). "Impact of early initiation of ezetimibe in patients with acute coronary syndrome: A systematic review and meta-analysis". Eur J Intern Med. 124: S0953-6205(24)00049-9. doi:10.1016/j.ejim.2024.02.004. PMID 38336550.
- ^ Awad K, Mikhailidis DP, Katsiki N, Muntner P, Banach M (March 2018). "Effect of Ezetimibe Monotherapy on Plasma Lipoprotein(a) Concentrations in Patients with Primary Hypercholesterolemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials". Drugs. 78 (4): 453–462. doi:10.1007/s40265-018-0870-1. PMID 29396832. S2CID 207489460.
- ^ Lee HY, Jun DW, Kim HJ, Oh H, Saeed WK, Ahn H, et al. (March 2019). "Ezetimibe decreased nonalcoholic fatty liver disease activity score but not hepatic steatosis". The Korean Journal of Internal Medicine. 34 (2): 296–304. doi:10.3904/kjim.2017.194. PMC 6406097. PMID 29551054.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ "Ezetimibe". Medline Plus. U.S. National Library of Medicine, National Institutes of Health, U.S. Department of Health and Human Services. 27 October 2014. Archived from the original on 5 July 2016. Retrieved 21 March 2018.
- ^ Takada T, Yamanashi Y, Konishi K, Yamamoto T, Toyoda Y, Masuo Y, et al. (February 2015). "NPC1L1 is a key regulator of intestinal vitamin K absorption and a modulator of warfarin therapy". Science Translational Medicine. 7 (275): 275ra23. doi:10.1126/scitranslmed.3010329. PMID 25696002. S2CID 5951911.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ "Ezetimibe - National Library of Medicine HSDB Database". toxnet.nlm.nih.gov. National Library of Medicine. Archived from the original on 12 June 2018. Retrieved 29 May 2018.
- ^ Basha SJ, Naveed SA, Tiwari NK, Shashikumar D, Muzeeb S, Kumar TR, et al. (June 2007). "Concurrent determination of ezetimibe and its phase-I and II metabolites by HPLC with UV detection: quantitative application to various in vitro metabolic stability studies and for qualitative estimation in bile". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 853 (1–2): 88–96. doi:10.1016/j.jchromb.2007.02.053. PMID 17442643.
{{cite journal}}: CS1 maint: overridden setting (link)
