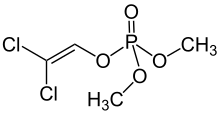
Dichlorvos (2,2-dichlorovinyl dimethyl phosphate, commonly abbreviated as an DDVP[1]) is an organophosphate widely used as an insecticide to control household pests, in public health, and protecting stored products from insects. The compound has been commercially available since 1961. It has become controversial because of its prevalence in urban waterways and the fact that its toxicity extends well beyond insects.[2] Since 1988, dichlorvos cannot be used as a plant protection product in the EU.[3]
| |

| |
| Names | |
|---|---|
| IUPAC name
2,2-Dichlorovinyl dimethyl phosphate
| |
| Other names
DDVP, Vapona[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.498 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H7Cl2O4P | |
| Molar mass | 220.97 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Use
editDichlorvos is effective against mushroom flies, aphids, spider mites, caterpillars, thrips, and whiteflies in greenhouses and in outdoor crops. It is also used in the milling and grain handling industries and to treat a variety of parasitic worm infections in animals and humans. It is fed to livestock to control botfly larvae in manure. It acts against insects as both a contact poison and an ingested poison. It is available as an aerosol and soluble concentrate. It is also used in pet flea collars and "no-pest strips" in the form of a pesticide-impregnated plastic; this material has been available to households since 1964 and has been the source of some concern, partly due to misuse.[4]
Properties
editDichlorvos is a colourless liquid[5] with aromatic odour.[6] Its density is 1.425 g/cm3 (23.35 g/in3) at 25 °C (77 °F),[6] melting point below −60 °C (−76 °F)[6] and a boiling point of 140 °C (284 °F) at 27 hPa.[6] Dichlorvos is soluble in water.[6]
Mechanism of action
editDichlorvos, like other organophosphate insecticides, inhibits acetylcholinesterase, associated with the nervous systems of insects. Evidence for other modes of action, applicable to higher animals, have been presented.[7][8] It is claimed to damage DNA of insects.[9]
Regulation
editThe United States Environmental Protection Agency has reviewed the safety data of dichlorvos several times.[10] In 1995 a voluntary agreement was reached with the supplier, Amvac Chemical Corporation, which restricted the use of dichlorvos in many, but not all, domestic uses, all aerial applications, and other uses.[11] Additional voluntary cancellations were implemented in 2006, 2008, and 2010. Major concerns focus on acute and chronic toxicity and the fact that this pesticide is prevalent in urban waterways.[12] A 2010 study found that each 10-fold increase in urinary concentration of organophosphate metabolites was associated with a 55% to 72% increase in the odds of ADHD in children.[13][14][15]
Between 2000 and 2013, thirty-one cases of acute dichlorvos pest strip-related illness were reported to the National Institute for Occupational Safety and Health (NIOSH) sentinel system. 65% of the 31 cases involved DDVP misuse contrary to instructions and safety labels.[16] Common violations included pest strip use in occupied, poorly-ventilated living areas (e.g., kitchens, bedrooms), lack of skin protection, cutting and tearing strips, and using a heater and fan to accelerate vapor dissemination from strips.
Production
editDichlorvos can be produced by dehydrochlorinating trichlorfon in an aqueous alkali at 40-50 °C. It is also produced by the reaction of trimethyl phosphate and chloral.[17] As of 1990, it was produced in Argentina, Brazil, Germany, India, Israel, Japan, the Republic of Korea, Mexico, the USA, Switzerland, Sweden, Spain, and the Netherlands.[18]
Environment
editDichlorvos enters the air, water, and soil when it is used and manufactured. It also can enter the environment when waste containing dichlorvos is disposed of in landfills. Dichlorvos is soluble in water, so it dissolves when it enters a body of water. Dichlorvos evaporates into the air easily, but is broken down by water vapor such as humidity. It does not bind to soil, but dichlorvos is broken down slower in soil than in the air. The broken down products are far less harmful than dichlorvos is. Dichlorvos is not stored in plants, animals, or humans.[19]
Safety
editPeople can be exposed to dichlorvos in the workplace by breathing it in, skin absorption, swallowing it, and eye contact. The Occupational Safety and Health Administration (OSHA) has set the legal limit (permissible exposure limit) for dichlorvos exposure in the workplace as 1 mg/m3 over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 1 mg/m3 over an 8-hour workday. At levels of 100 mg/m3, dichlorvos is immediately dangerous to life or health (IDLH).[20]
Treatment Technology
editDifferent physicochemical methods have been developed for the removal of DDVP from contaminated environments, and microbial degradation is regarded as a promising method to solve several harmful residuals caused by DDVP. The biodegradation mechanism of many OPs has been studied deeply, especially for the methyl parathion, whose degradation genes and enzymes were cloned and purified. There is a need to select more useful strains, since only a few bacteria have been studied thoroughly in relation to the functional enzymes and genes.[21]
Effects on humans
editSince it is an acetylcholinesterase inhibitor, symptoms of dichlorvos exposure include weakness, headache, tightness in chest, blurred vision, salivation, sweating, nausea, vomiting, diarrhea, abdominal cramps, eye and skin irritation, miosis (pupil constriction), eye pain, runny nose, wheezing, laryngospasm, cyanosis, anorexia, muscle fasciculation, paralysis, dizziness, ataxia, convulsions, hypotension (low blood pressure), and cardiac arrhythmias.[20]
It is also known to affect DNA replication in bacteria.[22]
| Dose | Organism | Time |
|---|---|---|
| 15 mg/m3 | rat | 4 h |
| 13 mg/m3 | mouse | 4 h |
| Dose | Organism | Route |
|---|---|---|
| 100 mg/kg | dog | oral |
| 61 mg/kg | mouse | oral |
| 10 mg/kg | rabbit | oral |
| 17 mg/kg | rat | oral |
Acute Effects
Dichlorvos is irritating to the skin. The substance may affect the nervous system by inhibiting cholinesterase. The effects of exposure may be delayed but a high level could be fatal. Medical observation is indicated[24]
Tests involving acute exposure of rats, mice, and rabbits have demonstrated that dichlorvos has high to extremely acute toxicity from oral or dermal exposure and extremely acute toxicity from inhalation[25]
Long-Term Effects
Prolonged contact with skin may cause dermatitis and skin sensitization.[24] If drinking water contaminated by dichlorvos is ingested long-term it may cause oral cancer.[25]
Reproductive/Developmental Effects
There is no information available on the reproductive or developmental effects of dichlorvos in humans. In studies conducted with rat models, birth defects in fetal rats were observed. Mouse models also demonstrated sperm abnormalities. However, studies with other animal subjects found no birth defects.[26]
Trivia
editDichlorvos is mentioned in John Brunner's science fiction novel The Sheep Look Up. One of the book's many vignettes tells of a woman who nearly dies, having taken barbiturates and gone to sleep in a closed room where a fly-killing strip doused with the material was placed.[27]
See also
edit- Metrifonate (converts into dichlorvos)
- Naled (can convert into dichlorvos)
References
edit- ^ a b "Dichlorvos". Haz-Map. U.S. National Library of Medicine. August 2015. Archived from the original on 2019-06-24. Retrieved 2015-10-13.
- ^ Das S (2013). "A review of Dichlorvos toxicity in fish". Current World Environment Journal. 8 (1). doi:10.12944/CWE.8.1.08.
- ^ "Which Pesticides are Banned in Europe?" (PDF). pan-europe.info. April 2008.
- ^ Gillett JW, Harr JR, Lindstrom FT, Mount DA, St Clair AD, Weber LJ (1972). "Evaluation of human health hazards on use of dichlorvos (DDVP), especially in resin strips". Residue Reviews. Vol. 44. pp. 115–59. doi:10.1007/978-1-4615-8491-9_6. ISBN 978-0-387-05863-4. PMID 4576326.
- ^ Entry on Dichlorvos. at: Römpp Online. Georg Thieme Verlag, retrieved 2014-02-07.
- ^ a b c d e Record of Dichlorvos in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2017-01-10.
- ^ Pancetti F, Olmos C, Dagnino-Subiabre A, Rozas C, Morales B (December 2007). "Noncholinesterase effects induced by organophosphate pesticides and their relationship to cognitive processes: implication for the action of acylpeptide hydrolase". Journal of Toxicology and Environmental Health, Part B. 10 (8): 623–30. Bibcode:2007JTEHB..10..623P. CiteSeerX 10.1.1.334.9406. doi:10.1080/10937400701436445. PMID 18049927.
- ^ Booth ED, Jones E, Elliott BM (December 2007). "Review of the in vitro and in vivo genotoxicity of dichlorvos". Regulatory Toxicology and Pharmacology. 49 (3): 316–26. doi:10.1016/j.yrtph.2007.08.011. PMID 17936460.
- ^ Espeland M, Irestedt M, Johanson KA, Akerlund M, Bergh JE, Källersjö M (January 2010). "Dichlorvos exposure impedes extraction and amplification of DNA from insects in museum collections". Frontiers in Zoology. 7: 2. doi:10.1186/1742-9994-7-2. PMC 2819063. PMID 20148102.
- ^ Mennear JH (June 1998). "Dichlorvos: a regulatory conundrum". Regulatory Toxicology and Pharmacology. 27 (3): 265–72. doi:10.1006/rtph.1998.1217. PMID 9693077.
- ^ "Dichlorvos (DDVP): Deletion of Certain Uses and Directions". U.S. Environmental Protection Agency: Office of Pesticide Programs. April 19, 1995. pp. 19580–19581.
Docket Control Number OPP-38511
- ^ Wines M (11 September 2014). "Pesticide Levels in Waterways Have Dropped, Reducing the Risks to Humans". The New York Times.
- ^ Brooks M (May 17, 2010). "Organophosphate Pesticides Linked to ADHD". Medscape.
- ^ Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG (June 2010). "Attention-Deficit/Hyperactivity Disorder and Urinary Metabolites of Organophosphate Pesticides". Pediatrics. 125 (6): e1270-7. doi:10.1542/peds.2009-3058. PMC 3706632. PMID 20478945.
- ^ Raeburn P (August 14, 2006). "Slow-Acting". Scientific American. Vol. 295, no. 2. p. 26. doi:10.1038/scientificamerican0806-26.
- ^ Pearson G. "CDC Warning on Misuse of Pest Strips". Wired. ISSN 1059-1028. Retrieved 2024-03-14.
- ^ Okoroiwu HU (August 2018). "Interdisciplinary toxicology". PMC 6829687.
{{cite web}}: Missing or empty|url=(help) - ^ to Humans IW (1991), "Dichlorvos", Occupational Exposures in Insecticide Application, and Some Pesticides, International Agency for Research on Cancer, PMID 1842580, retrieved 2024-03-14
- ^ "Dichlorvos | Public Health Statement | ATSDR". wwwn.cdc.gov. Retrieved 2024-03-14.
- ^ a b NIOSH Pocket Guide to Chemical Hazards. "#0202". National Institute for Occupational Safety and Health (NIOSH).
- ^ Zhang Y, Zhang W, Li J, Pang S, Mishra S, Bhatt P, Zeng D, Chen S (January 2021). "Emerging Technologies for Degradation of Dichlorvos: A Review". International Journal of Environmental Research and Public Health. 18 (11): 5789. doi:10.3390/ijerph18115789. ISSN 1660-4601. PMC 8199373. PMID 34071247.
- ^ "Preferential Effect of Dichlorvos(Vapona) on Bacteria Deficient in DNA Polymerase" (PDF). Cancer Research.
- ^ a b "Dichlorvos". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "ICSC 0690 - DICHLORVOS". www.ilo.org. Retrieved 2024-03-14.
- ^ a b "Dichlorvos" (PDF). EPA's integrated risk information system (IRIS). EPA. January 2000.
- ^ "Dichlorvos" (PDF). January 2000. Retrieved March 14, 2024.
- ^ Brunner J (1972). The Sheep Look Up. New York: Ballantine Books. p. 220. ISBN 978-0-06-010558-7. LCCN 72-79705.
External links
edit- Extension Toxicology Network - Pesticide Information Profiles - Dichlorvos (last maintained 1996)
- CDC - NIOSH Pocket Guide to Chemical Hazards
- Australian Pesticides & Veterinary Medicines Authority Chemical Review Program - Dichlorvos
- Material Safety Data Sheet (MSDS) for Dichlorvos
- Raeburn P (August 2006). "Slow-acting". Scientific American. 295 (2): 26. Bibcode:2006SciAm.295b..26R. doi:10.1038/scientificamerican0806-26. PMID 16866280.
- BBC News: Insecticide ban amid cancer fears
