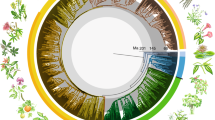
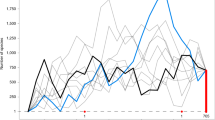
Abstract
The rise of angiosperms during the Cretaceous period is often portrayed as coincident with a dramatic drop in the diversity and abundance of many seed-free vascular plant lineages, including ferns1,2,3,4,5. This has led to the widespread belief that ferns, once a principal component of terrestrial ecosystems6, succumbed to the ecological predominance of angiosperms and are mostly evolutionary holdovers from the late Palaeozoic/early Mesozoic era. The first appearance of many modern fern genera in the early Tertiary fossil record implies another evolutionary scenario; that is, that the majority of living ferns resulted from a more recent diversification7,8,9,10. But a full understanding of trends in fern diversification and evolution using only palaeobotanical evidence is hindered by the poor taxonomic resolution of the fern fossil record in the Cretaceous11. Here we report divergence time estimates for ferns and angiosperms based on molecular data, with constraints from a reassessment of the fossil record. We show that polypod ferns (> 80% of living fern species) diversified in the Cretaceous, after angiosperms, suggesting perhaps an ecological opportunistic response to the diversification of angiosperms, as angiosperms came to dominate terrestrial ecosystems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Crane, P. R. in The Origin of Angiosperms and Their Biological Consequences (eds Friis, E. M., Chaloner, W. G. & Crane, P. R.) 107–144 (Cambridge Univ. Press, Cambridge, 1987)
Lidgard, S. & Crane, P. R. Angiosperm diversification and Cretaceous floristic trends: A comparison of palynofloras and leaf macrofloras. Paleobiology 16, 77–93 (1990)
Crane, P. R., Friis, E. M. & Pedersen, K. R. The origin and early diversification of angiosperms. Nature 374, 27–33 (1995)
Lupia, R., Lidgard, S. & Crane, P. R. Comparing palynological abundance and diversity: Implications for biotic replacement during the Cretaceous angiosperm radiation. Paleobiology 25, 305–340 (1999)
Nagalingum, N. S., Drinnan, A. N., Lupia, R. & McLoughlin, S. Fern spore diversity and abundance in Australia during the Cretaceous. Rev. Palaeobot. Palynol. 119, 69–92 (2002)
Niklas, K. J., Tiffney, B. H. & Knoll, A. H. Patterns in vascular land plant diversification. Nature 303, 614–616 (1983)
Smith, A. R. Comparison of fern and flowering plant distributions with some evolutionary interpretations for ferns. Biotropica 4, 4–9 (1972)
Lovis, J. D. Evolutionary patterns and processes in ferns. Adv. Bot. Res. 4, 229–415 (1977)
Rothwell, G. W. in Pteridology in Perspective (eds Camus, J. M., Gibby, M. & Johns, R. J.) 395–404 (Royal Botanic Gardens, Kew, 1996)
Collinson, M. E. in Pteridology in Perspective (eds Camus, J. M., Gibby, M. & Johns, R. J.) 349–394 (Royal Botanic Gardens, Kew, 1996)
Schneider, H. & Kenrick, P. An Early Cretaceous root-climbing epiphyte (Lindsaeaceae) and its significance for calibrating the diversification of polypodiaceous ferns. Rev. Palaeobot. Palynol. 115, 33–41 (2001)
Dilcher, D. L. Paleobotany: Some aspects of non-flowering and flowering plant evolution. Taxon 50, 697–711 (2001)
Friis, E. M., Pederson, K. R. & Crane, P. R. Fossil evidence of water lilies (Nymphaeales) in the Early Cretaceous. Nature 410, 357–360 (2001)
Soltis, D. E. et al. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. J. Linn. Soc. Bot. 133, 381–461 (2000)
Soltis, P. S., Soltis, D. E. & Chase, M. W. Angiosperm phylogeny inferred from multiple genes as a tool for comparative biology. Nature 402, 402–404 (1999)
Qiu, Y.-L. et al. The earliest angiosperms: Evidence from mitochondrial, plastid and nuclear genomes. Nature 402, 404–407 (1999)
Skog, J. E. Biogeography of Mesozoic leptosporangiate ferns related to extant ferns. Brittonia 53, 236–269 (2001)
Pryer, K. M. et al. Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature 409, 618–622 (2001)
Deng, S. Ecology of the Early Cretaceous ferns of Northeast China. Rev. Palaeobot. Palynol. 119, 93–112 (2002)
Sanderson, M. J. Estimating absolute rates of molecular evolution and divergence times: A penalized likelihood approach. Mol. Biol. Evol. 19, 101–109 (2002)
Pagel, M. & Lutzoni, F. in Biological Evolution and Statistical Physics (eds Lässig, M. & Valleriani, A.) 148–161 (Springer, Berlin, 2002)
Magallón, S. & Sanderson, M. J. Absolute diversification rates in angiosperm clades. Evolution 55, 1762–1780 (2001)
Wikström, N., Savolainen, V. & Chase, M. W. Evolution of the angiosperms: Calibrating the family tree. Proc. R. Soc. Lond. B 268, 2211–2220 (2001)
Benton, M. J. & Ayala, F. J. Dating the Tree of Life. Science 300, 1698–1700 (2003)
Soltis, P. S., Soltis, D. E., Savolainen, V., Crane, P. R. & Barraclough, T. G. Rate heterogeneity among lineages of tracheophytes: Integration of molecular and fossil data and evidence for molecular living fossils. Proc. Natl Acad. Sci. USA 99, 4430–4435 (2002)
Wikström, N. & Kenrick, P. Evolution of Lycopodiaceae (Lycopsida): Estimating divergence times from rbcL gene sequences by use of nonparametric rate smoothing. Mol. Phylogenet. Evol. 19, 177–186 (2001)
Kawai, H. et al. Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421, 287–290 (2003)
Smith, H. Phytochromes and light signal perception by plants—an emerging synthesis. Nature 407, 585–591 (2000)
Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (2001)
Sanderson, M. J. r8s: Inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19, 301–302 (2003)
Acknowledgements
We thank the Duke Biology systematics discussion group, especially P. Manos, for suggestions; F. Lutzoni, N. Nagalingum and A. R. Smith for comments on the manuscript; and M. Skakuj for the thumbnail sketches included in Fig. 1. This work was supported in part by grants from the National Science Foundation to H.S., K.M.P., R.C. and R.L.; by the Deep Time Research Coordination Network (NSF); and by the A.W. Mellon Foundation Fund to Duke University for Plant Systematics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Information
Provides our re-evaluation of the fossil record of major fern lineages and a synopsis of fossil fern constraints applied in this study (DOC 85 kb)
Supplementary Table 1
Provides rbcL and rps4 vouchers/citations and GenBank accession numbers for taxa not in Soltis et al. 2000. (XLS 51 kb)
Rights and permissions
About this article
Cite this article
Schneider, H., Schuettpelz, E., Pryer, K. et al. Ferns diversified in the shadow of angiosperms. Nature 428, 553–557 (2004). https://doi.org/10.1038/nature02361
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature02361
This article is cited by
-
A new fossil fern of the Dryopteridaceae (Polypodiales) from the mid-Cretaceous Kachin amber
Palaeobiodiversity and Palaeoenvironments (2023)
-
Bark traits affect epiphytic bryophyte community assembly in a temperate forest
Plant Ecology (2023)
-
An analysis of the current status and future prospects of Sri Lankan pteridophytes towards a new dimension
Biologia (2022)