Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies
BMJ 2015; 351 doi: https://doi.org/10.1136/bmj.h3978 (Published 12 August 2015) Cite this as: BMJ 2015;351:h3978- Russell J de Souza, assistant professor1234,
- Andrew Mente, associate professor125,
- Adriana Maroleanu, research volunteer2,
- Adrian I Cozma, medical student34,
- Vanessa Ha, doctoral student134,
- Teruko Kishibe, information specialist6,
- Elizabeth Uleryk, information specialist7,
- Patrick Budylowski, research volunteer4,
- Holger Schünemann, professor of medicine and department chair18,
- Joseph Beyene, professor of biostatistics12,
- Sonia S Anand, professor of medicine and epidemiology1258
- 1Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, ON, Canada
- 2Chanchlani Research Centre, McMaster University, Hamilton, ON, Canada
- 3Department of Nutritional Sciences, University of Toronto, Toronto, ON, Canada
- 4Clinical Nutrition and Risk Factor Modification Center, St Michael’s Hospital, Toronto, ON, Canada
- 5Population Health Research Institute, Hamilton Health Sciences, Hamilton, ON, Canada
- 6Scotiabank Health Sciences Library, St Michael’s Hospital, Toronto, ON, Canada
- 7Hospital Library and Archives, Hospital for Sick Children, Toronto, ON, Canada
- 8Department of Medicine, McMaster University, Hamilton, ON, Canada
- Correspondence to: S Anand, McMaster University, 1280 Main St W, MDCL-3204, Hamilton, ON L8N 3Z5, Canada anands{at}mcmaster.ca
- Accepted 15 July 2015
Abstract
Objective To systematically review associations between intake of saturated fat and trans unsaturated fat and all cause mortality, cardiovascular disease (CVD) and associated mortality, coronary heart disease (CHD) and associated mortality, ischemic stroke, and type 2 diabetes.
Design Systematic review and meta-analysis.
Data sources Medline, Embase, Cochrane Central Registry of Controlled Trials, Evidence-Based Medicine Reviews, and CINAHL from inception to 1 May 2015, supplemented by bibliographies of retrieved articles and previous reviews.
Eligibility criteria for selecting studies Observational studies reporting associations of saturated fat and/or trans unsaturated fat (total, industrially manufactured, or from ruminant animals) with all cause mortality, CHD/CVD mortality, total CHD, ischemic stroke, or type 2 diabetes.
Data extraction and synthesis Two reviewers independently extracted data and assessed study risks of bias. Multivariable relative risks were pooled. Heterogeneity was assessed and quantified. Potential publication bias was assessed and subgroup analyses were undertaken. The GRADE approach was used to evaluate quality of evidence and certainty of conclusions.
Results For saturated fat, three to 12 prospective cohort studies for each association were pooled (five to 17 comparisons with 90 501-339 090 participants). Saturated fat intake was not associated with all cause mortality (relative risk 0.99, 95% confidence interval 0.91 to 1.09), CVD mortality (0.97, 0.84 to 1.12), total CHD (1.06, 0.95 to 1.17), ischemic stroke (1.02, 0.90 to 1.15), or type 2 diabetes (0.95, 0.88 to 1.03). There was no convincing lack of association between saturated fat and CHD mortality (1.15, 0.97 to 1.36; P=0.10). For trans fats, one to six prospective cohort studies for each association were pooled (two to seven comparisons with 12 942-230 135 participants). Total trans fat intake was associated with all cause mortality (1.34, 1.16 to 1.56), CHD mortality (1.28, 1.09 to 1.50), and total CHD (1.21, 1.10 to 1.33) but not ischemic stroke (1.07, 0.88 to 1.28) or type 2 diabetes (1.10, 0.95 to 1.27). Industrial, but not ruminant, trans fats were associated with CHD mortality (1.18 (1.04 to 1.33) v 1.01 (0.71 to 1.43)) and CHD (1.42 (1.05 to 1.92) v 0.93 (0.73 to 1.18)). Ruminant trans-palmitoleic acid was inversely associated with type 2 diabetes (0.58, 0.46 to 0.74). The certainty of associations between saturated fat and all outcomes was “very low.” The certainty of associations of trans fat with CHD outcomes was “moderate” and “very low” to “low” for other associations.
Conclusions Saturated fats are not associated with all cause mortality, CVD, CHD, ischemic stroke, or type 2 diabetes, but the evidence is heterogeneous with methodological limitations. Trans fats are associated with all cause mortality, total CHD, and CHD mortality, probably because of higher levels of intake of industrial trans fats than ruminant trans fats. Dietary guidelines must carefully consider the health effects of recommendations for alternative macronutrients to replace trans fats and saturated fats.
Introduction
Recent high profile opinion pieces, informed by systematic reviews of randomized trials1 2 and prospective cohort studies,1 3 have called for a re-evaluation of dietary guidelines for intake and a re-appraisal of the effects of saturated fat on health; during this time public health efforts to remove trans fats from the food supply in several countries have intensified.
Saturated fats contribute about 10% of energy to the North American diet.4 5 The main sources of saturated fatty acids in the food supply are animal products, such as butter, cows’ milk, meat, salmon, and egg yolks, and some plant products such as chocolate and cocoa butter, coconut, and palm kernel oils. Previous meta-analyses of prospective cohort studies reported pooled relative risk estimates comparing extremes of intake of saturated fat of 1.07 (95% confidence interval 0.96 to 1.19; P=0.22) for coronary heart disease (CHD), 0.81 (0.62 to 1.05; P=0.11) for stroke, and 1.00 (0.89 to 1.11; P=0.95) for cardiovascular disease (CVD).6 Intervention trials have shown modest cardiovascular benefits of reducing intake of saturated fat while increasing intake of polyunsaturated fat,7 but most trials lasted only up to two years and examined surrogate outcomes. A meta-analysis of randomized trials suggested a 17% reduction in risk of CVD in studies that reduced saturated fat intake from about 17% to about 9% of energy (0.83, 0.72 to 0.96).8
Trans fats contribute about 1-2% of energy in the North American diet9 10 11 and are produced industrially through partial hydrogenation of liquid plant oils in the presence of a metal catalyst, vacuum, and high heat or can occur naturally in meat and dairy products, where ruminant animals biohydrogenate unsaturated fatty acids via bacterial enzymes. The major industrially produced trans fatty acids in the food supply are elaidic acid isomers, and the major ruminant derived trans fatty acid is vaccenic acid; both share the characteristic of having at least one double bond in the “trans-” rather than “cis-” configuration. A prior meta-analysis reported pooled relative risk estimates of CHD of 1.22 (95% confidence interval 1.08 to 1.38; P=0.002) for extremes of total intake of trans fats; 1.30 (0.80 to 2.14; P=0.29) for intake of industrially produced trans fats; and 0.93 (0.74 to 1.18; P=0.56) for intake of ruminant derived trans fats,12 suggesting that industrially produced trans fats might increase the risk of CHD, though this could also reflect the low levels of ruminant derived trans fats compared with the higher doses of industrially produced trans fats typically consumed in studies and available in the food supply.13
Dietary guidelines recommend that saturated fats should be limited to <10% (5-6% for those who would benefit from lowering of LDL cholesterol), and trans fats to <1% of energy or as low as possible,14 15 16 17 18 19 primarily to reduce risk of ischemic heart disease and stroke. To clarify controversies surrounding guidelines for saturated and trans fats for adults, we have extended and updated previous work to synthesize prospective associations between these fats and all cause mortality and type 2 diabetes (which have not been previously synthesized), separate estimates for risks of cardiovascular morbidity and mortality, and assess the confidence in the observational evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.20 21 22
Methods
This review was conducted in accordance with the WHO’s guideline development process,23 based on the Cochrane Collaboration approach24 and reported according to the MOOSE checklist.25
Data sources
We conducted independent searches for relevant observational studies assessing the association between saturated and/or trans fats and health outcomes up to 1 May 2015 (appendix 1 gives full details). This included searching Medline (from 1946), Embase (from 1974), Cochrane Central Registry of Controlled Trials (from 1996), Evidence Based Medicine Reviews (from 1996), and CINAHL (from 1983). Reference lists of retrieved articles and previous systematic and narrative reviews1 12 26 27 28 29 were hand searched. There were no language restrictions.
Study selection
Eligible studies included any observational study conducted in humans (such as prospective cohort, case-control, nested case-control, or case-cohort design) that reported a measure of association (such as hazard ratios or incident rate ratios for prospective studies; or odds ratios for retrospective studies) between intakes of saturated or trans fat, measured by self report or a biomarker, and all cause mortality, coronary heart disease, stroke, or type 2 diabetes, measured by self report, and/or confirmed by medical records or registry linkage. One reviewer assessed titles and abstracts of all studies identified through electronic searches. Potentially eligible studies were reviewed independently by a second reviewer, with discrepancies resolved by discussion; and when necessary, a senior author was consulted to reach consensus.
Data extraction
Pairs of authors independently extracted details of the study design, country of conduct, exposure and outcome assessment, participant characteristics, and statistical analyses, including adjustment for confounders, from included studies using pretested instruments (see appendix 1), with discrepancies resolved by discussion. Authors were contacted for additional data, when necessary. We used Plot Digitizer (http://plotdigitizer.sourceforge.net/) to extract numerical estimates from graphs.
Assessment of trans fats exposure methods
To assess the accuracy of measures of trans fats in studies that did not directly measure concentrations in blood or adipose tissues, we assessed the potential for misclassification. The lowest risk of misclassification was for those studies that used a food frequency questionnaire validated against multiple day prospective diet records or 24 hour recalls; directly measured adipose tissue trans fatty acids in a subset of the population; and analyzed dietary intake with an updated database of foods. A study was rated as at low risk of misclassification if it accomplished all three; moderate risk of misclassification) if it accomplished two of three; high risk of misclassification if it accomplished one of three; or at very high risk of misclassification if it did not accomplish any. For assessment of ruminant trans fats, the most common approach was to use the known nutrient composition from food tables for dairy and meat products to estimate ruminant trans fats and possibly supplemented by direct measurement with gas chromatography (see eTable 1 in appendix 2).
Study risk of bias
We used the Newcastle-Ottawa scale30 to assess the risk of bias of the included studies on the basis of selection of study groups, comparability of groups, and ascertainment of exposure(s) or outcome(s).
Grading of recommendations assessment, development, and evaluation (GRADE)
The GRADE approach was used to assess the confidence in the effect estimates derived from the body of evidence (quality of evidence) by outcome and produce evidence profiles.20 21 22 We limited the presentations of results in the main text to the synthesis of prospective cohort studies as these are considered the highest level of evidence for observational studies and thus were used for the GRADE assessments of confidence. Appendix 3 provides full details of designs that did not directly inform GRADE (that is, retrospective case-control studies and other studies not amenable to quantitative synthesis). All investigators discussed and reviewed evidence summaries and GRADE assessments, which were reviewed with the WHO Nutrition Guidance Expert Advisory Group (NUGAG) subgroup on diet and health as part of WHO’s guideline development process. Confidence in the estimate of each association was categorized into four levels, from very low to high.
Data synthesis and analysis
Statistical synthesis of effect estimates
The principal association measures were the risk ratios between extreme levels of intake (highest v lowest) for prospective studies and the odds ratio between extreme levels of exposure (highest v lowest) for retrospective studies. For each study, we calculated most adjusted (that is, the multivariable association measure with the highest number of covariates) and least adjusted (that is, the multivariable association measure with the fewest number of covariates) estimates and corresponding 95% confidence intervals for each outcome. We extracted both estimates to assess whether relevant confounders (such as smoking, age) and intermediate variables (such as LDL cholesterol, blood pressure) were captured in the statistical models. Analyses that adjust for potential confounders and intermediate variables will generally represent conservative estimates of the strength of the associations, and analyses that do not adjust for these will generally reflect the effect not only of exposure to fat but of other determinants of the health outcomes. We present both models to assess the impact of these variables on the reported association. When at least two studies provided data, we performed a DerSimonian and Laird random effects meta-analysis, which yields conservative confidence intervals around relative risks in the presence of heterogeneity.31 When three or fewer studies were combined, we also considered fixed effect estimates.
Heterogeneity
Heterogeneity was determined with Cochran’s Q test (significant at P<0.10), quantified with the I2 statistic (range from 0%-100%),32 and used to assess inconsistency as part of the GRADE assessment of evidence quality. If ≥10 studies were available33 34 and heterogeneity was substantial (I2>60% or PQ<0.10),32 we used meta-regression to explore heterogeneity by baseline year of study, continent of conduct, length of follow-up, median age of participants, proportion of smokers in the sample, amount of saturated fat in reference category, mean saturated or trans fat intake of the population, sex, α-linoleic acid, total polyunsaturated fat, adjustment for total energy, method and frequency of exposure assessment, risk of bias score, and adjustment for lipids or blood pressure (that is, causal intermediates).
Sensitivity
We carried out four a priori sensitivity analyses. We removed each single study from the meta-analyses and recalculated the summary association (the “leave one out” approach)35; removed studies with scores <7 on the Newcastle-Ottawa scale and recalculated the pooled association; included unpublished data on trans fats and CHD mortality (P Knekt, personal communication); and removed risk estimates imputed because of incomplete reporting. A study whose removal either pushed the significance level of the overall association from <0.05 to ≥0.05 (or vice versa), or altered the nominal effect size by 10% or more, was considered an influential outlier. At the request of the WHO’s Nutrition Guidelines Advisory Committee (10 July 2015), we performed two post hoc sensitivity analyses, limited to comparisons in which the estimated dietary intake of trans fatty acids was <1% of energy in the referent group and ≥1% of energy in the highest exposure category.
Publication bias
If ≥10 studies were available,36 we explored the possibility of publication bias by inspecting funnel plots and conducting Egger’s and Begg’s tests (each significant at P<0.10). If publication bias was suspected, results are shown without imputation and with “missing” studies imputed with Duval and Tweedie’s trim and fill method.37
Software
Primary summary analyses were carried out separately for each outcome with Review Manager, version 5.2 (Nordic Cochrane Center, Cochrane Collaboration, Copenhagen). Meta-regression and sensitivity analyses were undertaken with Stata, version 12.1 (StataCorp, College Station, TX).
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in the design and implementation of the study. There are no plans to involve patients in dissemination.
Results
Saturated fats and health outcomes
Literature flow
We identified 20 413 potentially eligible articles. After full text review, 41 primary reports of associations between saturated fats and health outcomes in prospective cohort studies (published between 1981 and 2014) provided 67 data points that contributed to the quantitative synthesis. Cohorts were enrolled from the United States (17 studies, 25 data points), the United Kingdom (four studies, six data points), Japan (four studies, nine data points), Sweden (four studies, seven data points), Israel (one study, four data points), Finland (three studies, four data points), Denmark (one study, four data points), Canada (one study, two data points), China (one study, one data point), Greece (one study, one data point), and Australia (one study, one data point) (fig 1⇓; appendix 2 provides full study characteristics in eTables 2-4 and scores on the Newcastle-Ottawa scale in eTables 5-6). The Seven Countries’ study, with cohorts enrolled from the US, Finland, the Netherlands, Italy, the former Yugoslavia (Serbia and Croatia), Greece, and Japan, is discussed separately as its design was not appropriate for pooling. Meta-regression analyses, summaries of results for case-control studies, nested case-control studies, dose-response/substitution relations, and studies that did not directly bear on the GRADE evidence table are presented in appendix 3 and eTables 7-10 in appendix 2.
Fig 1 PRISMA summary of evidence search and selection for saturated fat and health outcomes (up to 1 May 2015)
All cause mortality
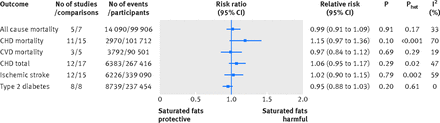
Six prospective investigations examined the association between intake of saturated fats and all cause mortality. The Seven Countries Study38 (12 763 men), which could not be included in the quantitative synthesis because of an incompatible association measure, reported large differences in intake across countries, from 3.9% (Tanushimaru, Japan) to 22.7% (East Finland). Over 25 years of follow-up, 5973 (47%) deaths were reported. In a multivariable regression model, a 5% increase in energy from saturated fats was associated with a 4.7% increase in age adjusted all cause mortality rate. For saturated fats and all cause mortality,39 40 41 42 43 the summary most adjusted multivariable risk ratio was 0.99 (95% confidence interval 0.91 to 1.09; P=0.91; I2=33%; Phet=0.17) (fig 2⇓; appendix 4 eFigure 1). Subgroup analyses or publication bias tests were not performed (<10 studies).
Fig 2 Summary most adjusted relative risks for saturated fat intake and all cause mortality, CHD mortality, CVD mortality, total CHD, ischemic stroke, and type 2 diabetes. All effect estimates are from random effects analyses. P value is for Z test of no overall association between exposure and outcome; Phet is for test of no differences in association measure among studies; I2 is proportion of total variation in study estimates from heterogeneity rather than sampling error
Fatal and total CHD and CVD
For saturated fats and CHD mortality,40 41 44 45 46 47 48 49 50 51 52 the summary most adjusted multivariable risk ratio was 1.15 (95% confidence interval 0.97 to 1.36; P=0.10; I2=70%; Phet<0.001) (fig 2⇑; appendix 4 eFigure 3). The summary least adjusted risk ratio was 1.20 (1.02 to 1.41; P=0.02; I2=74%; Phet<0.001) (appendix 4 eFigure 4). As risk estimates of four comparisons could not be directly extracted,47 48 50 we used the estimates reported in a previous meta-analysis.3 Removal of these four comparisons resulted in a summary risk ratio of 1.26 (0.98 to 1.62; P=0.07; I2=74%; Phet<0.001). Removal of the age group >60 in the study by Goldbourt and colleagues47 or the study by Pietinen and colleagues49 shifted the overall estimate to 1.20 (1.01 to 1.42; P=0.04; I2=68%; Phet<0.001), suggesting these two studies were influential outliers. For CVD mortality, the summary most adjusted multivariable risk ratio (five comparisons)39 43 53 was 0.97 (0.84 to 1.12; P=0.69; I2=19%; Phet=0.29) (fig 2⇑; appendix 4 eFigure 3).
For saturated fats and total CHD,44 49 51 54 55 56 57 58 59 60 61 62 the summary most adjusted multivariable risk ratio was 1.06 (95% confidence interval 0.95 to 1.17; P=0.29; I2=47%; Phet=0.02) (fig 2⇑; appendix 4 eFigure 5). As risk estimates from three comparisons54 58 could not be extracted, we used estimates reported in a previous meta-analysis.3 Removal of these three comparisons resulted in a summary risk ratio of 1.08 (0.97 to 1.20; P=0.18; I2=51%; Phet=0.01). The summary least adjusted relative risk was 1.12 (1.00 to 1.26; P=0.05; I2=63%; Phet<0.001) (appendix 4 eFigure 6). No study was an influential outlier.
For saturated fats and ischemic stroke,47 53 57 58 61 63 64 65 66 67 68 69 70 the summary most adjusted multivariable risk ratio was 1.02 (95% confidence interval 0.90 to 1.15; P=0.79; I2=59%; Phet=0.002) (fig 2⇑; appendix 4 eFigure 7). As risk estimates for four comparisons 47 54 58 could not be extracted, we used estimates reported in a previous meta-analysis.3 Removal of these four comparisons resulted in a summary risk ratio of 1.03 (0.89 to 1.19; P=0.68; I2=66%; Phet<0.001). The summary least adjusted risk ratio was 1.03 (0.91 to 1.16; P=0.65; I2=66%; Phet<0.001) (appendix 4 eFigure 8). No study was an influential outlier.
Type 2 diabetes
For saturated fats and type 2 diabetes,71 72 73 74 75 76 77 78the summary most adjusted multivariable risk ratio was 0.95 (95% confidence interval 0.88 to 1.03; P=0.20; I2=0%; Phet=0.61) (fig 2⇑; appendix 4 eFigure 9). The summary least adjusted risk ratio was 1.23 (0.98 to 1.52; P=0.07; I2=91%; Phet<0.001) (appendix 4 eFigure 10). No study was an influential outlier.
Trans fats and health outcomes
Literature flow
We identified 18 835 potentially eligible articles (fig 3⇓). After full text review, 20 primary reports of associations between total trans fats and the health outcomes in prospective cohort studies (published between 1996 and 2015) provided 28 data points that contributed to the quantitative synthesis. Cohorts were enrolled from the US (14 studies, 19 data points), Finland (four studies, six data points), China (one study, one data point), and the Netherlands (one study, two data points). One systematic review contributed one data point from a previously unpublished prospective cohort study,12 and one author provided updated unpublished data from the Finnish Mobile Health clinics (P Knekt, personal communication). Four primary reports of associations between industrial trans fats and the health outcomes (published between 1993 and 2013) provided four data points that contributed to the quantitative synthesis. Cohorts were enrolled from the US (one study, one data point), Finland (one study, one data point), the Netherlands (one study, one data point), and Norway (one study, one data point). Nine primary reports of associations between ruminant trans fats and the health outcomes (published between 1993 and 2015) provided 13 data points that contributed to the quantitative synthesis. Cohorts came from the US (five studies; five data points), Norway (one study, four data points), Finland (one study, one data point), Denmark (one study, two data points), and the Netherlands (one study, one data point). Appendix 2 shows full study characteristics: in eTable 11 for prospective cohort studies, eTable 12 for retrospective case-control studies, eTable 13 for nested case-control or case-cohort studies, and eTables 5 and 6 for scores on the Newcastle-Ottawa scale. Summaries of results for case-control studies, nested case-control studies, dose-response/substitution relations, and studies that did not inform the GRADE evidence table are presented in appendix 3. We use the term “total trans fats” to refer to the estimate of exposure to all trans fats, whether industrially produced or ruminant derived, and present specific associations of industrially produced and ruminant derived trans fats with health outcomes separately, when available. The specificity of trans fat measurement provided by each study is presented in appendix 2 eTables 1, 11, 12, and 13.
Fig 3 PRISMA summary of evidence search and selection for trans unsaturated fat and health outcomes (up to 1 May 2015)
All cause mortality
The pooled random effects most adjusted multivariable risk ratio of high versus low total intake of trans unsaturated fatty acid estimated from two published reports42 79 (two comparisons) including 2141 deaths in 20 346 individuals was 1.42 (95% confidence interval 1.04 to 1.94; P=0.03), with some evidence of heterogeneity between studies (I2=70%; Phet=0.07) appendix 4 eFigure 11). Because of the small number of studies, and the lower power to estimate τ for random effects analysis, we also performed a fixed effect meta-analysis, which results in a pooled association of 1.34 (1.16 to 1.56; P<0.001; I2=70%; Phet=0.07 (fig 4⇓, appendix 4 eFigure 12). The least adjusted estimate was 1.80 (1.57 to 2.07; P<0.001; I2=0%; Phet=0.71), presented in appendix 4 eFigures 13 and 14.
Fig 4 Summary most adjusted relative risks of total trans fat, industrial trans fat, and ruminant trans fat and all cause mortality, CHD mortality, total CHD, ischemic stroke, and type 2 diabetes. For total trans fats effect estimate for is fixed effect analysis; all others random effects analyses. P value is for Z test of no overall association between exposure and outcome; Phet is for test of no differences in association measure among studies; I2 is proportion of total variation in study estimates from heterogeneity rather than sampling error
Fatal and total CHD and CVD
For total trans fats and CHD mortality,44 49 51 52 80 the summary most adjusted multivariable risk ratio was 1.28 (95% confidence interval 1.09 to 1.50; P=0.003; I2=0%; Phet=0.66; fig 4⇑; appendix 4 eFigure 15; the least adjusted figure is shown in appendix 4 eFigure 16). Removal of the study by Pietinen and colleagues49 resulted in a relative risk of 1.20 (0.96 to 1.48; P=0.10; I2=0%; Phet=0.65). Addition of three unpublished comparisons from two cohorts, including updated data from one investigator (P Knekt, personal communication)12 weakened the estimate (1.22, 1.07 to 1.38; P=0.002; I2=0%; Phet=0.46) (appendix 4 eFigure 17; least adjusted eFigure 18).
For total trans fats and total CHD,44 49 51 55 59 80 the summary most adjusted multivariable risk ratio was 1.21 (1.10 to 1.33; P<0.001; I2=0%; Phet=0.43; fig 4⇑; appendix 2 eFigure 19; least adjusted in eFigure 20). We included data from one randomized trial55 as the report allowed a comparison of usual (about 2.5%) versus low (<1.1%) intake of trans fat at one year. Its removal did not alter the estimate of association (1.22, 1.08 to 1.38; P=0.002; I2=15%; Phet=0.32).
For total trans fats and ischemic stroke65 69 81 the summary most adjusted multivariable risk ratio was 1.07 (95% confidence interval 0.88 to 1.28; P=0.50) (fig 4⇑; appendix 4 eFigure 21 and least adjusted in eFigure 22). There was, however, considerable heterogeneity between studies (I2=67%; Phet=0.03).
Type 2 diabetes
For total trans fats and type 2 diabetes,72 73 74 75 76 82 the summary most adjusted multivariable risk ratio was 1.10 (95% confidence interval 0.95 to 1.27; P=0.21; I2=66%; Phet=0.01) (fig 4⇑; appendix 4 eFigure 23). Removal of one moderate quality study72 did not alter the estimate (1.14, 0.98 to 1.32; P=0.10; I2=63%; Phet=0.03). Pooling of minimally adjusted associations yielded a 28% increased risk of type 2 diabetes (1.28, 1.05 to 1.55; P=0.01; I2=87%; Phet<0.001; appendix 4 eFigure 24)
Industrially produced trans fats
The Norwegian Countries prospective cohort study83 (71 464 participants; 25.8 year follow-up) found no association between industrially produced trans fats from partially hydrogenated vegetable (PHVO) or fish oils (PHFO) and all cause mortality (11 980 deaths). The multivariable adjusted risk ratio was 0.96 (95% confidence interval 0.88 to 1.05; P=0.11 for trend) for high (≥1.65% of energy) versus low (<0.15% of energy) PHVO, and 1.00 (0.92 to 1.10; P=0.11 for trend) for high (≥2.35% of energy) versus low (<0.85% of energy) PHFO (fig 4⇑ shows the pooled risk ratio of PHVO and PHFO; I2=0% Phet=0.52). Two studies showed that industrially produced trans fats are associated with CHD mortality (1.18, 1.04 to 1.33; P=0.009; I2=0%; Phet=0.68; fig 4⇑; appendix 4 eFigures 25-28).49 83 Two other studies showed that industrially produced trans fats are associated with total CHD (1.42, 1.05 to 1.92; P=0.02; I2=34%; Phet=0.22)80 84 (fig 4⇑; appendix 4 eFigures 29-32). We did not find any prospective cohort studies of total intake of industrially produced trans fats and risk of ischemic stroke or type 2 diabetes.
Ruminant derived trans fats
In the Norwegian Countries prospective cohort study83 the multivariable adjusted risk ratio for all cause mortality was 1.04 (95% confidence interval 0.92 to 1.18; P=0.51; I2=4%; Phet=0.31) for the highest versus lowest categories of ruminant derived trans fats (fig 4⇑; appendix 4 eFigure 33-34). Two studies found no association between ruminant derived trans fats and CHD mortality49 83 (1.01, 0.71 to 1.43; P=0.95; I2=79%; Phet=0.01) (fig 4⇑; appendix 4 eFigure 35-36). Three studies found no association between ruminant derived trans fats and total CHD80 84 85 (0.93, 0.73 to 1.18; P=0.55; I2=46%; Phet=0.13) (fig 4⇑; appendix 4 eFigure 37-40). Removal of the study by Jakobsen and colleagues85 (in men) resulted in a pooled risk ratio of 0.83 (0.59 to 1.15; P=0.26; I2=28%; Phet=0.25), which met our definition of an “influential outlier.” Five studies found an inverse association between 16:1 n-7 trans-palmitoleic acid, principally derived from dairy, and type 2 diabetes82 86 87 88 89 (0.58, 0.46 to 0.74; P<0.001; I2=30%; Phet=0.22; fig 4⇑; appendix 4 eFigures 41-42). We did not find any prospective cohort studies of ruminant derived trans fats and risk of ischemic stroke.
GRADE confidence in estimates of association
For the GRADE confidence in estimates of association, we considered only prospective cohort studies because these are generally considered the highest level of observational study design.90 Overall, the certainty of the estimates for the association between saturated fats and all outcomes was very low, mainly because of low precision and high inconsistency (appendix 5). The certainty of the estimates for the association between total trans fats and total CHD and CHD mortality is moderate and very low to low for all others (appendix 6). Insufficient data were available to produce GRADE evidence profiles for industrially produced trans fats and ischemic stroke and ruminant derived trans fats and total CHD and ischemic stroke. These results suggest that further research is likely to have an important effect on our confidence in the estimation of association and could change the estimate.
Discussion
Principal findings
In this synthesis of observational evidence we found no clear association between higher intake of saturated fats and all cause mortality, CHD, CHD mortality, ischemic stroke, or type 2 diabetes among apparently healthy adults. Consumption of trans unsaturated fatty acids, however, was associated with a 34% increase in all cause mortality, a 28% increased risk of CHD mortality, and a 21% increase in the risk of CHD. Further, these data suggest that industrial trans fats confer a 30% increase in the risk of CHD events and an 18% increase in the risk of CHD mortality. No associations were observed for ruminant trans fat. Because of inconsistency in the included studies, we could not confirm an association between trans fats and type 2 diabetes and found no clear association between trans fats and ischemic stroke. This is the first meta-analysis of prospective observational studies examining associations of saturated and trans fats with all cause mortality and confirms the findings of five previous systematic reviews of saturated and trans fats and CHD.1 3 91 92 93
Saturated fats and health outcomes
All cause mortality
We found no association between saturated fat intake and all cause mortality, the Seven Countries’ Study notwithstanding. Controlled trials have shown that when saturated fats replaces carbohydrate in the diet, total and LDL cholesterol increase.94 Direct positive associations between total and LDL cholesterol concentrations and all cause and CHD mortality have been shown previously.95 96 97 We found no convincing lack of association with CHD mortality, the major contributor to total mortality. Studies of saturated fats and other major causes of death, such as colon98 and breast99 cancer, also generally fail to find significant associations. Foods high in saturated fats, particularly processed and red meats, however, have been associated with increased mortality100 101 102 and risk of cancer,103 104 105 though dairy foods are not consistently associated with cancers.106 A small body of evidence suggests that saturated fat increases risk of CVD and mortality among people with diabetes.107 108 This could relate to the LDL cholesterol raising effect of saturated fat and other metabolic consequences of insulin resistance among people with diabetes. In metabolic studies, saturated fat impairs insulin sensitivity and unsaturated fat improves glucose metabolism109; replacing saturated fat with monounsaturated fat improves lipoprotein and glycemic control in those with type 2 diabetes.110
CHD and CHD mortality
Saturated fats were not associated with total CHD, but we found a trend for association with CHD mortality. Risks associated with higher or lower intakes of macronutrients are sensitive to choice of replacement nutrient(s). In a pooled analysis of 11 prospective cohort studies (not included in our quantitative syntheses to avoid duplication of data), replacement of saturated fats with polyunsaturated fat reduced coronary risk by 13%,111 consistent with results of randomized controlled trials112 113 114; but replacement of saturated fat with monounsaturated fat or carbohydrate increased the risk of non-fatal myocardial infarction.111 In the Pooling Study cohorts, the primary sources monounsaturated fatty acids (MUFA) was animal fat, and some cohorts included trans fats in their definition of MUFA,111 so the effect of substitution of saturated fats with MUFA could reflect animal or processed food components not shared by plant sources of MUFA (such as olive or canola oils, avocado, and nuts). Carbohydrates in western diets are typically highly processed, high glycemic load foods, which could increase risk when they replace saturated fats.115 116 Inconsistent benefit was found for exchanging one food source of saturated fats for another,117 118 probably because many saturated fatty acids are common across different food sources.
Ischemic stroke
We found no association between saturated fats and risk of ischemic stroke, though the relative risk of stroke in the highest compared with the lowest categories of saturated fat exposure was reduced by 18% (0.82, 95% confidence interval 0.69 to 0.98) in studies conducted in Asian countries. The background saturated fat intake in North American studies was about 12% (range 9-16%), while in Asian studies it was about 9% (range 5-14%), with Japanese cohorts consistently <7%, suggesting that the effect of saturated fat might not be uniform across ethnic populations, intake levels, or possibly food sources.61 In the multi-center KANWU trial (n=162), a diet high in monounsaturated fat was associated with reduced blood pressure but a diet high in saturated fat was not.119
Type 2 diabetes
We found no association between total saturated fat intake and incident type 2 diabetes. Though saturated fats are believed to compromise insulin sensitivity,120 small randomized trials testing this relation yielded inconclusive results. In two larger trials, replacement of saturated fats with either MUFA or carbohydrate improved indices of glucose homeostasis.121 122 In the Women’s Health Initiative, reducing saturated fat intake from about 13% of energy to 9.5% did not reduce type 2 diabetes after 8.1 year follow-up.123 Positive associations have been reported between major sources of saturated fats, such as red and processed meat, and development of type 2 diabetes,124 125 while inverse associations have been reported for dairy products.126
A large (12 043 cases) case-cohort study (EPIC-InterAct),127 with nearly four million person years of follow-up, prospectively measured individual plasma phospholipid saturated fatty acids at a single time point. It found even-chain saturated fats were positively associated with incident type 2 diabetes (hazard ratios were 1.15 (95% confidence interval 1.09 to 1.22) for 14:0, myristic acid; 1.26 (1.15 to 1.37) for 16:0, palmitic acid; and 1.06 (1.00 to 1.13) for 8:0, stearic acid per 1 SD). By contrast, measured odd-chain saturated fats were inversely associated with incident type 2 diabetes (0.79 (0.73 to 0.85) for 15:0, pentadecanoic acid and 0.67 (0.63 to 0.71) for 17:0 heptadecanoic acid per 1 SD).
Odd-chain saturated fats seem to be relatively accurate biomarkers of dairy intake, whereas even chained saturated fats are poor markers of overall dietary intake.128 129 The findings for odd-chain saturated fats are consistent with an inverse association between dairy products and type 2 diabetes130; although residual confounding by other dairy components such as vitamin D, calcium, or fermentation products could explain this finding.130 131 Even-chain saturated fats (such as myristic, palmitic, and stearic acids) originate from de novo lipogenesis from carbohydrates and alcohol in liver or adipose tissue.132 133 Blood concentrations of these saturated fats, therefore, might not closely match dietary intake of saturated fats.134 The association of even-chain fatty acids with type 2 diabetes might reflect the effect of these other dietary components, or other mechanisms that also upregulate de novo lipogenic pathways. Palmitic acid, however, might activate inflammatory cytokines and pose specific lipotoxicity to pancreatic β cells.135
Trans fat and health outcomes
All cause mortality
Studies in the US and China were the first published cohort studies to report that trans fatty acids are associated with increased all cause mortality, though previous attempts had been made to model the impact of trans fats on mortality.136 137 In addition to CHD deaths,44 49 51 80 trans fats have been associated with sudden cardiac death138 and fatal colon139 and breast cancers.140 The World Cancer Fund panel, however, found insufficient evidence to implicate trans fats specifically for any type of cancer.106 More studies are needed to evaluate the contribution to non-cardiac mortality, which could be examined with data from existing cohorts.
CHD and CHD mortality
We found reliable and strong positive associations between trans fat intake and CHD and CHD mortality, consistent with several previous systematic reviews and meta-analyses.12 27 29 93 The effects on risk of heart disease are mediated via blood lipids and pro-inflammatory processes.141 142 143 144 145 146 147 148 Our finding that a 2% increase in energy from trans fats is associated with a 25% increased risk of CHD and 31% increase in CHD mortality (appendix 2 eTables 14-17) is consistent with conclusions of two previous meta-analyses.29 93
Ischemic stroke
The two prospective studies that assessed the association between trans fats and ischemic stroke yielded inconsistent results. One study in men showed no association with stroke65; the other, in women, showed a positive association in those who did not take aspirin.69 Further the association with trans fats was significant only for lacunar stroke, with a trend for hemorrhagic stroke but not for stroke of cardioembolic origin. A nested case-control study conducted within the Women’s Health Initiative Observational Study (WHI-OS) with 10 year follow-up149 found no association between serum total trans 16:1, 18:1, or 18:2 and ischemic stroke; these results were not included in our quantitative synthesis because the different trans fats reported could not be classified as “total” or strictly “industrial” or “ruminant” derived. The association with risk of stroke requires further study.
Type 2 diabetes
We found no association between trans fats and type 2 diabetes, though the interpretation of this finding is complicated by heterogeneity. Inconsistency has also been noted in randomized trials of the effects on glucose homeostasis.150 Two cohort studies reporting strong associations between trans fats and type 2 diabetes73 74 were generally similar to those that did not with respect to measures of exposures, outcomes, and most covariates, except that the three studies that failed to show an association adjusted for fiber and magnesium,72 75 76 which might protect against diabetes,151 152 while the two studies that showed an association73 74 did not. Pooling estimates without adjustment for magnesium and fiber yields a 16% increased risk of type 2 diabetes with high trans fat intake (four studies; risk ratio 1.16, 95% confidence interval 0.95 to 1.41; I2=82%; Phet<0.001); when we limited analysis to the three studies with no serious risks of bias,73 74 76 this became a 28% increased risk (three studies; 1.28, 1.16 to 1.41; P<0.001; I2=0%; Phet=0.87).
The role of trans-palmitoleic acid in prevention of type 2 diabetes could represent an important new direction for fatty acid research. It is important to note, however, that the exposure levels to this nutrient are typically low. In the three included studies, trans-palmitoleic acid represented <1% of total fatty acid intake, with the mean reported exposure level varying about eightfold across cohorts (mean 0.06% to 0.49% of plasma phospholipid fatty acids), with considerable variability within the cohort (SD ranging from 0.03% to 0.20%). Nevertheless, the protective associations with type 2 diabetes are quite consistent (I2=30%) and compatible with a 26-54% reduction in risk across an estimated threefold intake range. The biology of a potential protective effect of trans-palmitoleic acid against type 2 diabetes could relate to its ability to mimic the role of cis-palmitoleic acid, which is protective against diabetes in animals.153
Industrially produced v ruminant derived trans fats
Consistent with the findings of a previous meta-analysis of observational studies,12 our study, which included recent data from a large Norwegian study,83 found that industrially produced, but not ruminant derived, trans fats are associated with risk of CHD. This might reflect a true difference between sources or might be a function of consumption levels. Ruminant derived trans fats are consumed at relatively low levels in most populations; in the studies included in our present analysis, the average intake of industrially produced trans fats was about 2.5-fold that of ruminant derived trans fats (mean energy intakes of about 1.8% (range about 0.3-3.7%) and 0.7% (0.6-0.8%), respectively). The greater range of intake of industrially produced trans fats in cohort studies provides greater statistical power for detection of associations.
Two quantitative syntheses of randomized controlled trials of ruminant derived trans fats and biomarkers of cardiovascular risk arrived at opposite conclusions. Brouwer and colleagues pooled six randomized controlled trials of ruminant derived trans fats and 29 of industrially produced trans fats and found that both had similar impacts on LDL:HDL cholesterol when they were consumed across an equivalent intake range (0.7-6.6% of energy),13 which supports the notion that the lack of association of ruminant derived trans fats with cardiovascular outcomes in the present and previous analyses12 is related to their lower intake levels. Gayet-Boyer and colleagues, however, pooled 13 randomized controlled trials (including all of those included by Brouwer and colleagues) and found no linear association between ruminant derived trans fats and LDL:HDL cholesterol or total:HDL cholesterol across a dose range of 0.1-4.2% of energy.154 The reasons for this discrepancy are unclear but could relate to differences in the approaches taken to the quantitative synthesis (such as study weighting, regression modeling) or inclusion criteria (such as minimum duration of studies, acceptable choice of comparison arms). Further research is required to assess the impact of ruminant derived versus industrially produced trans fats on health outcomes; but the best available observational evidence suggests that at the reported intake levels in the included studies, ruminant trans fats do not increase the risk of developing the health outcomes reviewed here.
In support of the importance of exposure levels, case-control studies in Costa Rica and Australia found that the association between total trans fats and CHD was attenuated after removal of industrially produced trans fats from the food supply,155 156 which resulted in lower levels of consumption of total trans fats, primarily consisting of ruminant derived trans fats. Case-control studies have shown a strong association between trans-18:2 isomers,155 157 158 159 160 161 abundant in partially hydrogenated oils, and CHD (six studies, seven comparisons; multivariable odds ratio 1.82, 95% confidence interval 1.14 to 2.90; P=0.01; I2=77%; Phet<0.001; appendix 4 eFigure 43) but no significant association between trans-18:1 isomers,155 157 158 159 160 161 162—derived principally from partially hydrogenated oils, but also found in ruminant foods—and CHD (seven studies, eight comparisons; 1.19, 0.93 to 1.51; P=0.16; I2=59%; Phet=0.02; appendix 4 eFigure 44).
A community based 10 year prospective cohort study of older adults (the Cardiovascular Health Study, US)149 measured the association between phospholipid concentrations of specific trans fatty acids found chiefly in prepared foods163 (trans-16:1n9, trans-18:2 (trans/cis-18:2; cis/trans-18:2; and trans/trans-18:2), and trans-18:1) and all cause death and deaths from CHD and CVD. Circulating trans/trans- and trans/cis-18:2 were generally harmful, but variation existed across classes, with a noteworthy lack of association for trans-18:1, the major component of partially hydrogenated vegetable oils. Of public health importance is that commercially produced trans fatty acids other than trans-18:1 can remain in the food supply even after removal of partially hydrogenated oils, via vegetable oil deodorization and high temperature frying.164 165 166 Future work is needed to assess the public health importance of this residual risk.
Methodological issues related to measuring intake of a nutrient at such low levels (<1% of energy), and the complexity of parsing specific trans fatty acids into “industrial” or “ruminant” sources, also decreases our confidence in the results for ruminant derived trans fats. With the phasing out of industrially produced partially hydrogenated oils in several countries, future prospective studies might be better positioned to assess the effects of ruminant derived trans fats on health. Based on currently available data from prospective cohort studies, ruminant derived trans fats are not associated with risk of CHD, though it is uncertain whether this a true biological difference or a function of their lower levels of intake during the periods of study.
In a post hoc sensitivity analysis, we estimated the effect of total trans fats on CHD mortality and total CHD at levels similar to those reported in the studies of ruminant trans fats included in the analysis, to help to assess whether the generally low exposure levels to ruminant trans fatty acids were driving the lack of association observed for these outcomes in the ruminant trans fat analysis. To do so, we pooled the multivariable relative risks for quantiles that most closely approximated a 0.8% of energy increase from the referent category for total trans fat and CHD mortality; and a 1.2% of energy increase from the referent category for total trans fat and CHD. In this sensitivity analysis, for total trans fats and CHD mortality, the risk ratio was 1.02 (five studies, six comparisons; 95% confidence interval 0.90 to 1.16; P=0.73; Phet=0.25; I2=24%; appendix 4 eFigure 45; exposure estimates in appendix 2 eTable 20) or 1.03 when we added unpublished studies (seven studies, nine comparisons; 0.95 to 1.12; P=0.45; Phet=0.36; I2=9%; appendix 4 eFigure 46). For total trans fats and CHD, the risk ratio was 1.17 (six studies, seven comparisons; 1.07 to 1.29; P<0.001; Phet=0.41; I2=1%; appendix 4 eFigure 47).
Consistency across observational designs
Findings in prospective cohorts were generally consistent with those from case-control studies, which found that higher exposure to trans fats (whether measured by food frequency questionnaire or biomarker) was associated with a 51% increased odds of CHD (odds ratio 1.51, 95% confidence interval 1.08 to 2.09; P=0.01; I2=75%; Phet<0.001). This was attenuated and no longer significant when we restricted the synthesis to high quality studies (1.37, 0.78 to 2.41; P=0.28; I2=78%; Phet<0.001) (appendix 4 eFigures 45-47). Inclusion of nested case-control studies167 168 169 170 171 172 in meta-analyses of prospective studies of total trans fats and CHD mortality (appendix 4 eFigure 48), CHD (appendix 4 eFigure 49), type 2 diabetes (appendix 4 eFigure 50) did not substantively alter the pooled association from the association derived from prospective cohort studies. Nested case-control studies with biomarkers of saturated fat intake (such as erythrocyte or adipose tissue) collected before occurrence of disease, though few in number, consistently found that people with highest levels of exposure to saturated fat were at increased risk of CHD mortality, total CHD, and type 2 diabetes, and these methods of exposure measurement are less subject to bias. Pooling of prospective cohorts with nested case-control studies of saturated fats resulted in a borderline significant association with CHD mortality but not total CHD or type 2 diabetes (appendix 4 eFigures 51-53). Prospective studies with repeated biomarker assessments will advance knowledge in this area.
Strengths and weaknesses of the study
This study has several strengths. First, we assessed confidence in the estimates with GRADE to facilitate guideline development. Second, studies were identified through a systematic search of the literature, augmented with manual searches of reference lists of published papers and systematic reviews. Third, the quantitative synthesis focused on studies measuring comparable outcomes with similar designs, reducing methodological heterogeneity.
There were, however, important limitations related to evidence synthesis and quality. First, meta-analytic techniques depend on the availability of conceptually similar and combinable effect estimates across studies. If such estimates are not available, the ability to pool all available and relevant data in a meaningful way is compromised, and the pooled estimate of effect might be suboptimal. Notably, in our evidence synthesis, the positive association between saturated fat and total mortality observed in the Seven Countries’ Study38 could not be combined with other association estimates because the β coefficient could not be directly converted into an estimate of relative risk. The GRADE approach offers a methodological advance in evaluating the quality of the body of evidence in a transparent fashion, and thus a “non-combinable” estimate can still inform our judgment of the presence, strength, and direction of an effect. Therefore, because of this inconsistency, we document the inconsistency between this finding (positive) and that of the pooled prospective cohort studies (null), and rate the confidence we have in a true quantitative “null” association as “very low.”
Second, observational studies cannot provide causal evidence of an effect of saturated or trans fatty acids on the development of health outcomes examined; they can describe only associations. Measurement error is often serious in epidemiologic studies of diet and disease, which can bias such associations towards the null. Major limitations of the included studies are described in appendix 2 eTables 3a and 3b (Newcastle-Ottawa evaluations) and in the footnotes to the GRADE tables (appendices 5 and 6). These include unrepresentative cohorts or a vaguely defined cohort sampling frame; misclassification of exposure from inaccurate measurement tools (selection and exposure measurement biases); failure to account for major confounders such as age, socioeconomic status, smoking, total energy, or family history (non-comparability biases); and lack of validated outcome measures or insufficient study duration to observe a high number of events (outcome assessment biases). Additionally, random error can attenuate the observed associations between trans fats and health outcomes and also explain the lack of association between saturated fat and health outcomes. This error can arise from several sources, including residual confounding, recall bias, and exposure misclassification.
The reviewed studies typically relied on food frequency questionnaires, 24 hour recalls, or seven day food records, each of which has serious limitations in their ability to accurately capture long term dietary fat intake. Tissue levels of saturated fat are not always valid measures of dietary saturated fat, and associations based on these exposure measures are difficult to interpret because of shared endogenous and exogenous sources. Exposure measurement error is potentially more serious with trans fatty acids, though analytical methods for determining trans fatty acid content of foods and tissues, and differentiating ruminant derived from industrially produced trans fatty acids, has evolved considerably since 1980.173 It is difficult to classify trans fat isomers as ruminant or industrial because of shared food sources, and self reported intakes can be incorrect because of outdated food databases and the rapidly changing trans fat content of foods. These limitations are especially important given that during the timeframe of the studies reviewed most countries were making major efforts to remove trans fats from the food supply.
Third, several investigators adjusted for changes in risk factors on the causal pathway between diet and disease, serum lipids and blood pressure, which attenuates relations between saturated or trans fats and the outcomes. The validity of use of “most adjusted” models, which account both for potential confounders and causal intermediates, has been debated.174 175 Models adjusted for potential confounders and intermediate variables underestimate associations because of over-controlling for the effect of causal intermediates; unadjusted models overestimate associations because estimates reflect other determinants of the health outcomes. Comparability across studies is compromised when different studies include different sets of confounders. To assess the potential impact of over-adjustment, we assessed “intermediately adjusted models”—that is, those that adjusted for the most relevant confounders (smoking, age, sex, and total energy) but not potential causal intermediates (blood pressure or anti-hypertensive drugs, serum lipids or lipid lowering drugs)—for associations for which we had a high number of studies: saturated fat and cardiovascular outcomes. In these sensitivity analyses, the adjusted risk ratio was 1.21 (95% confidence interval 0.93 to 1.58; eight studies) for saturated fat and CHD mortality; 1.05 (0.93 to 1.19; 11 studies) for saturated fat and total CHD; and 0.87 (0.76 to 1.00, two studies) for saturated fat and ischemic stroke. These figures would not meaningfully change our conclusions based on the fully adjusted models.
Fourth, although we carried out extensive subgroup analyses with meta-regression, the substantial heterogeneity present in most analyses for saturated fats remains unexplained.
Fifth, because of a small number of cohorts, dose-response relations, or differences between specific sources of saturated or trans fatty acids on health outcomes, were not robustly quantified. We had insufficient data to perform robust subgroup analyses for trans fatty acids associations. In post hoc sensitivity analyses presenting highest versus lowest intakes only in those studies where the referent group had an estimated trans fat intake <1% of energy, or a highest intake ≥1% of energy, provided results consistent with the main analyses (appendix 2 eTables 18 and 19; appendix 4 eFigures 54-62).
Strengths and weaknesses in relation to other studies
This is the seventh systematic review and meta-analysis of observational studies of saturated and/or trans fats and health outcomes in the past 10 years.1 3 12 91 93 141 176 Our work updates and corroborates previous systematic reviews and meta-analyses of observational studies that have also failed to find associations between saturated fat and CVD,1 total CHD,1 3 91 93 fatal CHD,1 93 and stroke3; positive associations between trans fat and total CHD1 12 91 93 141 and fatal CHD12 93; and no association with type 2 diabetes.176 A Cochrane review of randomized trials of reduced saturated fats and cardiovascular events found a 17% reduced risk with lower saturated fat intake (risk ratio 0.83, 95% confidence interval 0.72 to 0.96; 13 studies with 53 300 participants; moderate GRADE).8 Methodological advantages of randomized controlled trials over prospective cohort studies include the balancing of known and unknown confounders and better measurement and finer control of dietary fat levels.
Limitations of comparison of extremes
Our a priori research question was to examine the effect on the health outcomes of higher compared with lower saturated fat, which we did by comparing highest and lowest intake estimates. Such a comparison, however, obscures the importance of reciprocal and possibly heterogeneous decreases in other macronutrients that accompany high saturated or trans fat intakes. Thus, an overarching consideration is that the effect estimate of higher intakes of saturated or trans fats on health outcomes is linked to the nutrient that it replaces. Most studies in the present review did not explicitly model the effects of nutrient substitution, but when total energy, protein, and alcohol are covariates in the multivariable model, coefficients for fat reflect substitution of saturated or trans fat for carbohydrate. Indeed, carbohydrate energy was typically lowest in those in the highest intakes of saturated and trans fat. Common sources of carbohydrate in typically studied populations were highly processed high glycemic load foods,115 which can increase risk of CHD independently of saturated and trans fats through different metabolic pathways; likely attenuating the observed associations between these fats and outcomes.177
Replacement of saturated fats by high quality carbohydrate
The analysis of data from the largest prospective study to examine carbohydrate quality, as measured by glycemic index, suggests that replacement of saturated fat with high glycemic index carbohydrate increased the risk of CVD, but replacement with low glycemic index carbohydrate (such as whole fruits, vegetables, pulses, and grains) decreased risk.116
Replacement of saturated fats by unsaturated fats
In cohort studies that have directly modeled substitution effects, replacement of saturated fat by polyunsaturated fat (with a corresponding increase in polyunsaturated:saturated (P:S) ratio conferred the greatest reduction in risk of CVD111; though these studies did not distinguish between n-3 and n-6 fatty acids as the replacement choice. Several intervention studies that have replaced saturated fat with polyunsaturated fats achieved relatively high P:S ratios (>1.0 to about 2.5) through replacement of saturated fat with predominantly soybean (n-6 linoleic) and marine oils (n-3 eicosapentaenoic and docosahexaenoic acids from sardines). At these levels significant CHD benefits were seen,112 113 114 consistent with the finding that favorable effects of diets with reduced saturated fat on cardiovascular risk might depend on a significant reciprocal increase in polyunsaturated fat92 or high quality carbohydrate from whole fruits, vegetables, pulses, and grains, which tend to have a lower glycaemic index.116 In a meta-analysis of cohort studies, replacement of 5% of energy from saturated fat with linoleic acid (n-6 PUFA) was associated with a 9% lower risk of CHD events (risk ratio 0.91, 95% confidence interval 0.87 to 0.96; 13 studies with 310 602 participants) and a 13% lower risk of CHD deaths (0.87, 0.82 to 0.94).178 A re-analysis of the Sydney Diet Heart Study and updated meta-analysis, however, found no benefit and possible harm associated with replacement of saturated fat by linoleic acid (hazard ratio 1.33 (95% confidence interval 0.99 to 1.79) for CHD death and 1.27 (0.98 to 1.65) for CVD) in secondary prevention trials.179 Replacement of saturated fat with monounsaturated fat or carbohydrate was not associated with significant reduction in CHD risk but was associated with a small increase in risk of non-fatal myocardial infarction.115 The relative risks associated with different saturated fats or their food sources were not importantly different, with the exception of a single study that noted replacement of saturated fat from meat with saturated fat from dairy decreased risk of CVD.118 Other components of these foods, however, could also be responsible for these effects.
Replacement of trans fats by carbohydrate
In the two studies that directly assessed the impact on type 2 diabetes of replacement of carbohydrate with trans fat, replacement of 1% of energy from carbohydrate with trans fatty acids was associated with a 23% increased risk (hazard ratio 1.23, 95% confidence interval 1.02 to 1.48)73 and replacement of 2% of energy from carbohydrate with trans fatty acids was associated with a 39% increased risk (1.39, 1.15 to 1.67).72 In the two studies that directly assessed the impact of replacement of carbohydrate with trans fats,64 68 replacement was associated with either no increased risk of stroke in men (risk ratio 0.86, 0.55 to 1.32, per 2% of energy)64 or a small but significant increase risk of stroke in older post-menopausal women (1.08, 1.004 to 1.16, per 2 g of intake).68
Replacement of trans fat by unsaturated fats
Using data from two of the largest prospective cohort studies, Mozaffarian and Clarke28 reported the adjusted risk ratio of CHD for isocaloric replacement of 2% of energy from trans unsaturated fatty acids with saturated fatty acids, monounsaturated fatty acids, or polyunsaturated fatty acids. They found that replacement of 2% of energy from trans fats with saturated fat would reduce risk by 17% (risk ratio 0.83, 95% confidence interval 0.75 to 0.93). The reductions in risk were 21% (0.79, 0.70 to 0.88) for replacement with monounsaturated fat and 24% (0.76, 0.67 to 0.85) for replacement with polyunsaturated fat. In the present analysis, we found no new evidence that would substantially alter these risks.
Meaning of the study
This systematic review and meta-analysis of evidence from large generally well designed observational studies does not support a robust association of saturated fats with all cause mortality, CHD, CHD mortality, ischemic stroke, or diabetes in healthy individuals; but the choice of comparison nutrient (n-6 and/or n-3 PUFA, MUFA, refined or high quality carbohydrate) must be carefully considered. Few observational studies, however, modeled the effect of replacing saturated or trans fats with other nutrients. In large prospective studies, when polyunsaturated fats replace saturated fats, risk of CHD is reduced but not when MUFA or carbohydrate is the replacement choice. Higher, compared with lower, intakes of trans fats are associated with a 20-30% increased risk of all cause mortality, CHD and CHD mortality, regardless of choice of replacement nutrient, but associations with type 2 diabetes and stroke are unclear. The association seems to be most consistently driven by industrially produced trans fats, probably because of their higher intakes among participants during the follow-up periods of the included studies. Dietary guidelines for saturated and trans fatty acids must carefully consider the effect of replacement nutrients.
Unanswered questions and future research
Several questions could not be answered by our review. First, do different sources (for example, animal v plant) and chain lengths (odd v even) of saturated fat have different effects on health, particularly with respect to risk of diabetes? The current evidence reviewed suggests that dairy fats, specifically odd chained saturated fatty acids, might be protective against type 2 diabetes; but, apart from recommendations for broad sources of fatty acids (such as dairy v plant v animal flesh), it is not feasible to separate different types of saturated fats with respect to food choices because the foods contain a combination of several saturated fats. Second, what is the impact of saturated fats consumed in the context of diverse background diets on health? Notably, the association between certain foods and CHD cannot be predicted solely by their content of total saturated fats because individual saturated fats might have different cardiovascular effects, and major food sources of saturated fat contain other constituents that could influence risk of CHD. Third, are there meaningful differences in the choice of polyunsaturated fat—for instance, n-3 or n-6—that replaces saturated (or trans) fats in the diet? Current evidence suggests that either group of polyunsaturated fats provide similar benefit. Fourth, is the reported protective effect of trans-palmitoleic acid for type 2 diabetes robust, and, if so, does the apparent benefit extend to cardiovascular disease outcomes? Fifth, do threshold levels of ruminant trans fatty acid intakes exist, above which cardiovascular risk increases in a similar fashion to that seen with industrial trans fatty acids? Finally, what should be the “gold” standard for measurement of fatty acid intake? Development of reliable and valid methods of assessing fatty acid intakes in large longitudinal cohort studies with sufficient follow-up to observe clinical events and deaths must remain a priority to improve the quality of the evidence on which dietary advice is based.
What is already known on this topic
Contrary to prevailing dietary advice, authors of a recent systematic review and meta-analyses claim that there is no excess cardiovascular risk associated with intake of saturated fat, and the US has recently taken policy action to remove partially hydrogenated vegetable oils from its food supply
Population health guidelines require a careful review and assessment of the evidence of harms of these nutrients, with a focus on replacement nutrients
What this study adds
This study reviewed prospective observational studies and assessed the certainty of the associations with GRADE methods
There was no association between saturated fats and health outcomes in studies where saturated fat generally replaced refined carbohydrates, but there was a positive association between total trans fatty acids and health outcomes
Dietary guidelines for saturated and trans fatty acids must carefully consider the effect of replacement nutrients
Notes
Cite this as: BMJ 2015;351:h3978
Footnotes
We are grateful to Viranda Jayalath (University of Toronto) for his assistance developing the data abstraction forms. We thank Paul Knekt, Anthony Hanley, and Ingrid Santaren for providing data, and Hannia Campos, and Kay-tee Khaw for clarifying aspects of their studies; Christine Neilson and Natalie Campbell for their assistance with the literature search; Michael Zulyniak for assistance with preparing the manuscript for publication; and the members of the WHO Nutrition Guidance Advisory Group (NUGAG) Subgroup on Diet and Health for their helpful comments on the draft results. WHO agreed to the publication of this systematic review in a scientific journal because it serves as the background evidence review for updating WHO guidelines on saturated and trans fatty acids and should therefore be available widely. We appreciate the helpful comments of peer reviewers Arne Atrup, Ronald Krauss, JM Chardigny, and Evangeline Mantzioris, which have greatly improved the quality of the manuscript.
Contributors: Study concept and design: RJdeS, SSA, JB, AMe. Development and implementation of literature search strategy: EU, TK. Acquisition of data, including review of literature search results and data abstraction: RJdeS, EU, TK, AMe, AMa, AIC, VH, PB. Analysis and interpretation of data: RJdeS, AMe, SSA, JB, HS. Drafting of the manuscript: RJdeS, AMe, VH, AIC. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: RJdeS, JB. Administrative, technical, and material support: EU, TK, AM. Study supervision: SSA, JB. RJdeS is guarantor.
Funding: This study was funded by WHO, which defrayed costs associated with preparing the draft manuscript, including information specialist and technical support and article retrieval costs. This systematic review was presented by RJdeS at the 5th Nutrition Guidelines Advisory Group (NUGAG) meeting in Hangzhou, China (4-7 March, 2013), the 6th NUGAG meeting in Copenhagen, Denmark (21-24 Oct, 2013), and the 7th NUGAG meeting in Geneva, Switzerland (9-12 Sept, 2014); and via skype during the 8th NUGAG meeting in Fukuoka, Japan (9-12 June, 2015). WHO covered travel and accommodation costs for RJdeS to attend these meetings. The research questions for the review were discussed and developed by the WHO Nutrition Guidance Expert Advisory Group (NUGAG) Subgroup on Diet and Health and the protocol was agreed by the WHO NUGAG Subgroup on Diet and Health; however, neither WHO nor the WHO NUGAG Subgroup on Diet and Health had any role in data collection or analysis.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: RJdeS has received a Canadian Institutes for Health Research (CIHR) postdoctoral fellowship. VH has received a Province of Ontario graduate scholarship and research support from the Canadian Institutes of Health Research (CIHR). AIC has received a Province of Ontario graduate scholarship.
Ethical approval: Not required.
Transparency statement: RJdeS affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies are disclosed.
Data sharing: The full dataset and statistical code are available from the corresponding author.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.