Abstract
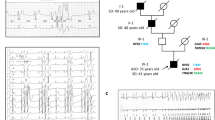
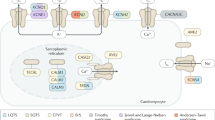
Ventricular fibrillation causes more than 300, 000 sudden deaths each year in the USA alone1,2. In approximately 5–12% of these cases, there are no demonstrable cardiac or non-cardiac causes to account for the episode, which is therefore classified as idiopathic ventricular fibrillation (IVF)3,4,5,6. A distinct group of IVF patients has been found to present with a characteristic electrocardiographic pattern7,8,9,10,11,12,13,14,15. Because of the small size of most pedigrees and the high incidence of sudden death, however, molecular genetic studies of IVF have not yet been done. Because IVF causes cardiac rhythm disturbance, we investigated whether malfunction of ion channels could cause the disorder by studying mutations in the cardiac sodium channel gene SCN5A. We have now identified a missense mutation, a splice-donor mutation, and a frameshift mutation in the coding region of SCN5A in three IVF families. We show that sodium channels with the missense mutation recover from inactivation more rapidly than normal and that the frameshift mutation causes the sodium channel to be non-functional. Our results indicate that mutations in cardiac ion-channel genes contribute to the risk of developing IVF.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kannel, W. B., Cupples, A. & D'Agostino, R. B. Sudden death risk in overt coronary heart disease: the Framingham study. Am. Heart J. 113, 799–804 (1987).
Willich, S.et al. Circadian variation in the incidence of sudden cardiac death in the Framingham heart study population. Am. J. Cardiol. 60, 801–806 (1987).
Trappe, H. J.et al. Prognosis of patients with ventricular tachycardia and ventricular fibrillation: role of the underlying etiology. J. Am. Coll. Cardiol. 12, 166–174 (1988).
Viskin, S. & Belhassen, B. Idiopathic ventricular fibrillation. Am. Heart J. 120, 661–671 (1990).
Wichter, T., Breithardt, G. & Borggrefe, M. in Cardiac Arrhythmia: Mechanism, Diagnosis, and Management (eds Podrid, P. J. & Kowey, P. R.) 1219–1238 (Williams & Wilkins, Maryland, 1995).
Martini, B.et al. Ventricular fibrillation without apparent heart disease: description of six cases. Am. Heart. J. 118, 1203–1209 (1989).
Brugada, J., Brugada, R. & Brugada, P. Right bundle branch block, ST segment elevation in leads V1–V3: a marker for sudden death in patients without demonstrable structural heart disease. Circulation 96, I151 (1997).
Brugada, P. & Brugada, J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J. Am. Coll. Cardiol. 20, 1391–1396 (1992).
Brugada, P. & Brugada, J. Further characterization of the syndrome of right bundle branch block, ST segment elevation, and sudden cardiac death. J. Cardiovasc. Electrophysiol. 8, 325–331 (1997).
Brugada, J., Brugada, P. & Brugada, R. Ajmaline unmasks right bundle branch block-like and ST segment elevation in V1–V3 in patients with idiopathic ventricular fibrillation. PACE 19, 599 (1996).
Miyanuma, H., Sakurai, M. & Odaka, H. Two cases of idiopathic ventricular fibrillation with interesting electrocardiographic findings. Kokyu to Junkan 41, 287–291 (1993).
Aizawa, Y.et al. Idiopathic ventricular fibrillation and bradycardia-dependent intraventricular block. Am. Heart J. 126, 1473–1474 (1993).
Sumiyoshi, M.et al. Acase of idiopathic ventricular fibrillation with incomplete right bundle branch block and persistent ST segment elevation. Jpn Heart J. 34, 661–666 (1993).
Bjerregaard, P., Gussak, I., Kotar, S. L., Gessler, J. E. & Janosik, D. Recurrent syncope in a patient with prominent J wave. Am. Heart J. 127, 1426–1430 (1994).
Miyazaki, T.et al. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J. Am. Coll. Cardiol. 27, 1061–1070 (1996).
Tung, R. T., Shen, W., Hammil, S. C. & Gersh, E. J. Idiopathic ventricular fibrillation in out-of-hospital cardiac arrest survivors. PACE 17, 1405–1411 (1994).
Cobb, L. A. in Hurst's The Heart (eds Schlant, R. C. & Alexander, R. W.) 8th edn. 947–957 (McGraw Hill, New York, 1994).
Wang, Q., Li, Z., Shen, J. & Keating, M. T. Genomic organization of the human SCN5A gene encoding the cardiac sodium channel. Genomics 34, 9–16 (1996).
Rogers, J. C., Qu, Y., Tanada, T. N., Scheuer, T. & Catterall, W. A. Molecular determinants of high affinity binding of α-scorpion toxin and sea anemone toxin in the S3–S4 extracellular loop in domain IV of the Na channel α subunit. J. Biol. Chem. 271, 15950–15962 (1996).
Hoffman, E. P., Lehmann-Horn, F. & Rudel, R. Overexcited or inactive: ion channels in muscle disease. Cell 80, 681–686 (1995).
Yang, N. & Horn, R. Evidence for voltage-dependent S4 movement in sodium channels. Neuron 15, 213–218 (1995).
Shapiro, M. B. & Senapathy, P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 15, 7155–7174 (1987).
Wang, Q.et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell 80, 805–811 (1995).
Wang, Q.et al. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum. Mol. Genetics 4, 1603–1607 (1995).
Bennett, P. B., Yazawa, K., Makita, N. & George, A. L. J Molecular mechanism for an inherited cardiac arrhythmia. Nature 376, 683–685 (1995).
Dumaine, R.et al. Multiple mechanisms of Na+ channel-linked long-QT syndrome. Circulation Res. 78, 916–992 ((1996).
Stuhmer, W.et al. Structural parts involved in activation and inactivation of the sodium channel. Nature 339, 597–603 (1989).
Krishnan, S. C. & Antzelevitch, C. Sodium channel block produces opposite electrophysiological effects in canine ventricular epicardium and endocardium. Circulation Res. 69, 277–291 (1991).
Krishnan, S. C. & Antzelevitch, C. Flecainide-induced arrhythmia in canine ventricular epicardium. Phase 2 reentry? Circulation 87, 562–572 (1992).
Yan, G. X. & Antzelevitch, C. Cellular basis for the electrocardiographic J wave. Circulation 93, 372–379 (1996).
Acknowledgements
We thank H. Hartmann for the wild-type SCN5A construct; M. Sanguinetti and P.Spector for help with electrophysiological analysis of the 1-bp deletion; and P. Szafranski, J. T. Bricker, M. Scheinman, A.L. Beaudet, A. Bradley and X. Qu for help and advice. This work ws supported by a Grant-In-Aid from the American Heart association, by the AHA, Northeast Ohio Affiliate (G.E.K.), and the Deutsche Forschungsgemeinschaft (E.S.-B.), and by grants from the NIH and Bristol-Myers Squibb (M.T.K.), The Texas Children's Hospital Foundation Chair in Pediatric Cardiac Research and NIH grants (J.A.T.), the Carolien Weiss Law Grant for Research in Molecular Medicine (Q.W.), the Abercrombie Cardiology Fund of Texas Children's Hospital (Q.W.), and a Scientist Development Award from the American Hearth Association (Q.W.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Q., Kirsch, G., Zhang, D. et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature 392, 293–296 (1998). https://doi.org/10.1038/32675
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/32675
This article is cited by
-
Kardiogenetik in Deutschland – ein (Rück‑)Blick
Herzschrittmachertherapie + Elektrophysiologie (2024)
-
GPD1L-A306del modifies sodium current in a family carrying the dysfunctional SCN5A-G1661R mutation associated with Brugada syndrome
Pflügers Archiv - European Journal of Physiology (2024)
-
HiPSC-derived cardiomyocyte to model Brugada syndrome: both asymptomatic and symptomatic mutation carriers reveal increased arrhythmogenicity
BMC Cardiovascular Disorders (2023)
-
Screening, diagnosis and follow-up of Brugada syndrome in children: a Dutch expert consensus statement
Netherlands Heart Journal (2023)
-
Role of Ca2+ in healthy and pathologic cardiac function: from normal excitation–contraction coupling to mutations that cause inherited arrhythmia
Archives of Toxicology (2023)